Teniloxazine
Teniloxazine
Jump to navigation
Jump to search
 | |
| Clinical data | |
|---|---|
| Synonyms | Y-8894 |
| Routes of administration | Oral |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
IUPAC name
| |
| CAS Number |
|
PubChem CID |
|
| ChemSpider |
|
| UNII |
|
| Chemical and physical data | |
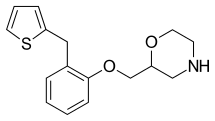
| Formula | C16H19NO2S |
| Molar mass | 289.393 g/mol |
| 3D model (JSmol) |
|
SMILES
| |
InChI
| |
.mw-parser-output .nobold{font-weight:normal} (verify) | |
Teniloxazine (Lucelan, Metatone), also known as sufoxazine and sulfoxazine, is a drug which is marketed in Japan.[1] Though initially investigated as a neuroprotective and nootropic agent for the treatment of cerebrovascular insufficiency in the 1980s,[2][3][4][5][6][7][8][9] it was ultimately developed and approved as an antidepressant instead.[10] It acts as a potent norepinephrine reuptake inhibitor, with fair selectivity over the serotonin and dopamine transporters, and also behaves as an antagonist of the 5-HT2A receptor.[7][10][11]
See also[edit]
- Bifemelane
- Indeloxazine
- Viloxazine
References[edit]
^ C. R Ganellin; D. J Triggle; F.. Macdonald (1997). Dictionary of pharmacological agents. CRC Press. p. 1905. ISBN 978-0-412-46630-4. Retrieved 27 October 2011..mw-parser-output cite.citation{font-style:inherit}.mw-parser-output q{quotes:"""""""'""'"}.mw-parser-output code.cs1-code{color:inherit;background:inherit;border:inherit;padding:inherit}.mw-parser-output .cs1-lock-free a{background:url("//upload.wikimedia.org/wikipedia/commons/thumb/6/65/Lock-green.svg/9px-Lock-green.svg.png")no-repeat;background-position:right .1em center}.mw-parser-output .cs1-lock-limited a,.mw-parser-output .cs1-lock-registration a{background:url("//upload.wikimedia.org/wikipedia/commons/thumb/d/d6/Lock-gray-alt-2.svg/9px-Lock-gray-alt-2.svg.png")no-repeat;background-position:right .1em center}.mw-parser-output .cs1-lock-subscription a{background:url("//upload.wikimedia.org/wikipedia/commons/thumb/a/aa/Lock-red-alt-2.svg/9px-Lock-red-alt-2.svg.png")no-repeat;background-position:right .1em center}.mw-parser-output .cs1-subscription,.mw-parser-output .cs1-registration{color:#555}.mw-parser-output .cs1-subscription span,.mw-parser-output .cs1-registration span{border-bottom:1px dotted;cursor:help}.mw-parser-output .cs1-hidden-error{display:none;font-size:100%}.mw-parser-output .cs1-visible-error{font-size:100%}.mw-parser-output .cs1-subscription,.mw-parser-output .cs1-registration,.mw-parser-output .cs1-format{font-size:95%}.mw-parser-output .cs1-kern-left,.mw-parser-output .cs1-kern-wl-left{padding-left:0.2em}.mw-parser-output .cs1-kern-right,.mw-parser-output .cs1-kern-wl-right{padding-right:0.2em}
^ Anami K, Yamamoto Y, Setoguchi M (February 1985). "[Pharmacological studies on sufoxazine (Y-8894). (I) Effects on experimental amnesia in mice]". Nihon Yakurigaku Zasshi. Folia Pharmacologica Japonica (in Japanese). 85 (2): 71–7. doi:10.1254/fpj.85.71. PMID 2859238.
^ Izumi N, Yasuda H (October 1985). "[Pharmacological studies on sufoxazine (Y-8894). (II). Anti-anoxic effect]". Nihon Yakurigaku Zasshi. Folia Pharmacologica Japonica (in Japanese). 86 (4): 323–8. doi:10.1254/fpj.86.323. PMID 4085932.
^ Usa T, Morimoto Y, Fukuda T, Anami K, Setoguchi M, Maruyama Y (October 1986). "[Pharmacological studies on Y-8894. (III). Its effect on the abnormal electrocorticogram induced by destruction of the internal capsule]". Nihon Yakurigaku Zasshi. Folia Pharmacologica Japonica (in Japanese). 88 (4): 289–97. doi:10.1254/fpj.88.289. PMID 3491778.
^ Yasuda H, Izumi N, Nakanishi M, Anami K, Maruyama Y (November 1986). "[Pharmacological studies on Y-8894. (IV). Ameliorative effect on a cerebral energy metabolism disorder induced by KCN]". Nihon Yakurigaku Zasshi. Folia Pharmacologica Japonica (in Japanese). 88 (5): 363–7. doi:10.1254/fpj.88.363. PMID 3817653.
^ Anami K, Yamamoto Y, Setoguchi M, Maruyama Y (March 1987). "[Pharmacological studies on Y-8894. (V) Effect on learning and memory in intact and experimentally amnesic rats]". Nihon Yakurigaku Zasshi. Folia Pharmacologica Japonica (in Japanese). 89 (3): 145–53. doi:10.1254/fpj.89.145. PMID 2884174.
^ ab Setoguchi M, Takehara S, Sakamori M, Anami K, Maruyama Y (July 1987). "[Pharmacological studies on Y-8894 (VI). The effect on monoamine uptake and turnover in mouse brain]". Nihon Yakurigaku Zasshi. Folia Pharmacologica Japonica (in Japanese). 90 (1): 41–9. doi:10.1254/fpj.90.41. PMID 2443434.
^ Yasumatsu H, Yamamoto Y, Takamuku H, et al. (December 1987). "[Pharmacological studies on Y-8894. (VII). Effects on transient cerebral ischemia-induced amnesia in rats]". Nihon Yakurigaku Zasshi. Folia Pharmacologica Japonica (in Japanese). 90 (6): 321–30. doi:10.1254/fpj.90.321. PMID 3443414.
^ Anami K, Setoguchi M, Senoh H (August 1988). "[Pharmacological studies on Y-8894. (VIII). Effects on learning and memory in the radial maze task in mice]". Nihon Yakurigaku Zasshi. Folia Pharmacologica Japonica (in Japanese). 92 (2): 113–8. doi:10.1254/fpj.92.113. PMID 3224898.
^ ab Ogura C, Kishimoto A, Kunimoto N, et al. (May 1987). "Clinical pharmacology of a new antidepressant, Y-8894 in healthy young and elderly volunteers". British Journal of Clinical Pharmacology. 23 (5): 537–43. doi:10.1111/j.1365-2125.1987.tb03089.x. PMC 1386189. PMID 3593624.
^ "Therapeutic Agent for Attention-Deficit Hyperactivity Disorder - Google Patents". Retrieved October 27, 2011.
Categories:
- Drugs not assigned an ATC code
- Antidepressants
- Morpholines
- Thiophenes
- Phenol ethers
(window.RLQ=window.RLQ||).push(function(){mw.config.set({"wgPageParseReport":{"limitreport":{"cputime":"0.924","walltime":"1.107","ppvisitednodes":{"value":6045,"limit":1000000},"ppgeneratednodes":{"value":0,"limit":1500000},"postexpandincludesize":{"value":427445,"limit":2097152},"templateargumentsize":{"value":5681,"limit":2097152},"expansiondepth":{"value":16,"limit":40},"expensivefunctioncount":{"value":3,"limit":500},"unstrip-depth":{"value":1,"limit":20},"unstrip-size":{"value":34799,"limit":5000000},"entityaccesscount":{"value":3,"limit":400},"timingprofile":["100.00% 767.429 1 -total"," 57.12% 438.325 1 Template:Drugbox"," 38.95% 298.893 1 Template:Infobox"," 23.65% 181.522 1 Template:Reflist"," 20.83% 159.892 25 Template:Navbox"," 12.82% 98.403 16 Template:Unbulleted_list"," 11.45% 87.899 9 Template:Cite_journal"," 8.32% 63.884 1 Template:Cite_book"," 6.41% 49.157 1 Template:Antidepressants"," 5.93% 45.521 1 Template:Navbox_with_collapsible_groups"]},"scribunto":{"limitreport-timeusage":{"value":"0.321","limit":"10.000"},"limitreport-memusage":{"value":5149066,"limit":52428800}},"cachereport":{"origin":"mw1263","timestamp":"20181215042510","ttl":1900800,"transientcontent":false}}});});{"@context":"https://schema.org","@type":"Article","name":"Teniloxazine","url":"https://en.wikipedia.org/wiki/Teniloxazine","sameAs":"http://www.wikidata.org/entity/Q7699855","mainEntity":"http://www.wikidata.org/entity/Q7699855","author":{"@type":"Organization","name":"Contributors to Wikimedia projects"},"publisher":{"@type":"Organization","name":"Wikimedia Foundation, Inc.","logo":{"@type":"ImageObject","url":"https://www.wikimedia.org/static/images/wmf-hor-googpub.png"}},"datePublished":"2011-10-27T17:04:52Z","dateModified":"2017-06-23T06:59:55Z","image":"https://upload.wikimedia.org/wikipedia/commons/1/12/Teniloxazine.svg","headline":"chemical compound"}(window.RLQ=window.RLQ||).push(function(){mw.config.set({"wgBackendResponseTime":122,"wgHostname":"mw1333"});});

