Naftidrofuryl
Naftidrofuryl
Jump to navigation
Jump to search
 | |
| Clinical data | |
|---|---|
| Trade names | Praxilene |
AHFS/Drugs.com | International Drug Names |
| Routes of administration | Oral |
| ATC code |
|
| Legal status | |
| Legal status |
|
Pharmacokinetic data | |
| Metabolism | Hepatic |
| Elimination half-life | 1 - 3.5 hours |
| Identifiers | |
IUPAC name
| |
| CAS Number |
|
PubChem CID |
|
| ChemSpider |
|
| UNII |
|
| ChEMBL |
|
| ECHA InfoCard | 100.045.960 |
| Chemical and physical data | |
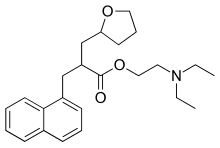
| Formula | C24H33NO3 |
| Molar mass | 383.524 g/mol |
| 3D model (JSmol) |
|
SMILES
| |
InChI
| |
.mw-parser-output .nobold{font-weight:normal} (verify) | |
Naftidrofuryl (INN), also known as nafronyl or as the oxalate salt naftidrofuryl oxalate or nafronyl oxalate, is a vasodilator used in the management of peripheral and cerebral vascular disorders. It is also claimed to enhance cellular oxidative capacity. The drug act as a selective antagonist of 5-HT2 receptors (with action as an inverse agonist of the 5-HT2A receptor specifically characterized).[1][2][3] Naftidrofuryl is also licensed for the treatment of intermittent claudication due to peripheral arterial disease.
Naftidrofuryl is marketed under a variety of trade names, including Artocoron, Azunaftil, Di-Actane, Dusodril, Enelbin, Frilix, Gevatran, Iridus, Iridux, Luctor, Nafti, Naftilong, Naftodril, Nafoxal, Praxilene, Sodipryl retard, and Vascuprax.
Historically, it has been used to treat sudden idiopathic hearing loss and acute tinnitus.[4]
Naftidrofuryl may be effective for relieving the pain of muscle cramps.[5]
Adverse Effects[edit]
Naftidrofuryl has been associated with nausea, abdominal pain and rash. Rarely, hepatitis and liver failure have been reported.[6]
See also[edit]
- Ketanserin
- Sarpogrelate
References[edit]
^ Peter Lanzer; Eric J. Topol (20 December 2013). Pan Vascular Medicine: Integrated Clinical Management. Springer. pp. 1394–. ISBN 978-3-642-56225-9..mw-parser-output cite.citation{font-style:inherit}.mw-parser-output q{quotes:"""""""'""'"}.mw-parser-output code.cs1-code{color:inherit;background:inherit;border:inherit;padding:inherit}.mw-parser-output .cs1-lock-free a{background:url("//upload.wikimedia.org/wikipedia/commons/thumb/6/65/Lock-green.svg/9px-Lock-green.svg.png")no-repeat;background-position:right .1em center}.mw-parser-output .cs1-lock-limited a,.mw-parser-output .cs1-lock-registration a{background:url("//upload.wikimedia.org/wikipedia/commons/thumb/d/d6/Lock-gray-alt-2.svg/9px-Lock-gray-alt-2.svg.png")no-repeat;background-position:right .1em center}.mw-parser-output .cs1-lock-subscription a{background:url("//upload.wikimedia.org/wikipedia/commons/thumb/a/aa/Lock-red-alt-2.svg/9px-Lock-red-alt-2.svg.png")no-repeat;background-position:right .1em center}.mw-parser-output .cs1-subscription,.mw-parser-output .cs1-registration{color:#555}.mw-parser-output .cs1-subscription span,.mw-parser-output .cs1-registration span{border-bottom:1px dotted;cursor:help}.mw-parser-output .cs1-hidden-error{display:none;font-size:100%}.mw-parser-output .cs1-visible-error{font-size:100%}.mw-parser-output .cs1-subscription,.mw-parser-output .cs1-registration,.mw-parser-output .cs1-format{font-size:95%}.mw-parser-output .cs1-kern-left,.mw-parser-output .cs1-kern-wl-left{padding-left:0.2em}.mw-parser-output .cs1-kern-right,.mw-parser-output .cs1-kern-wl-right{padding-right:0.2em}
^ I.K. Morton; Judith M. Hall (6 December 2012). Concise Dictionary of Pharmacological Agents: Properties and Synonyms. Springer Science & Business Media. pp. 147–. ISBN 978-94-011-4439-1.
^ Aly, Saida Abdel Regal; Hossain, Murad; Bhuiyan, Mohiuddin Ahmed; Nakamura, Takashi; Nagatomo, Takafumi (2009). "Assessment of Binding Affinity to 5-Hydroxytryptamine 2A (5-HT2A) Receptor and Inverse Agonist Activity of Naftidrofuryl: Comparison With Those of Sarpogrelate". Journal of Pharmacological Sciences. 110 (4): 445–450. doi:10.1254/jphs.09124FP. ISSN 1347-8613.
^ "DER ARZNEIMITTELBRIEF: Infusionstherapie beim idiopathischen Hörsturz? Dextran Dextran Hörsturz Hydroxyethylstärke Pentoxifyllin Pentoxifyllin Procain Taprosten". www.der-arzneimittelbrief.de.
^ Katzberg HD, Khan AH, So YT (February 2010). "Assessment: Symptomatic treatment for muscle cramps (an evidence-based review): Report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology". Neurology. 74 (8): 691–6. doi:10.1212/WNL.0b013e3181d0ccca. PMID 20177124.
^ Brayfield, A, ed. (14 January 2014). "Naftidrofuryl Oxalate". Martindale: The Complete Drug Reference. Pharmaceutical Press. Retrieved 6 August 2014.
This drug article relating to the cardiovascular system is a stub. You can help Wikipedia by expanding it. |
Categories:
- 5-HT2 antagonists
- 5-HT2A antagonists
- Vasodilators
- Naphthalenes
- Tetrahydrofurans
- Carboxylate esters
- Amines
- Cardiovascular system drug stubs
(window.RLQ=window.RLQ||).push(function(){mw.config.set({"wgPageParseReport":{"limitreport":{"cputime":"0.776","walltime":"0.930","ppvisitednodes":{"value":4824,"limit":1000000},"ppgeneratednodes":{"value":0,"limit":1500000},"postexpandincludesize":{"value":304101,"limit":2097152},"templateargumentsize":{"value":4070,"limit":2097152},"expansiondepth":{"value":14,"limit":40},"expensivefunctioncount":{"value":3,"limit":500},"unstrip-depth":{"value":1,"limit":20},"unstrip-size":{"value":17510,"limit":5000000},"entityaccesscount":{"value":3,"limit":400},"timingprofile":["100.00% 636.208 1 -total"," 63.70% 405.279 1 Template:Drugbox"," 46.60% 296.477 1 Template:Infobox"," 22.48% 143.043 1 Template:Reflist"," 20.37% 129.620 18 Template:Navbox"," 12.66% 80.541 16 Template:Unbulleted_list"," 12.07% 76.821 2 Template:Cite_book"," 9.06% 57.650 1 Template:Serotonergics"," 6.17% 39.270 1 Template:Infobox_drug/chemical_formula"," 5.50% 35.000 2 Template:Cite_journal"]},"scribunto":{"limitreport-timeusage":{"value":"0.255","limit":"10.000"},"limitreport-memusage":{"value":4680889,"limit":52428800}},"cachereport":{"origin":"mw1224","timestamp":"20181213234418","ttl":1900800,"transientcontent":false}}});});{"@context":"https://schema.org","@type":"Article","name":"Naftidrofuryl","url":"https://en.wikipedia.org/wiki/Naftidrofuryl","sameAs":"http://www.wikidata.org/entity/Q425867","mainEntity":"http://www.wikidata.org/entity/Q425867","author":{"@type":"Organization","name":"Contributors to Wikimedia projects"},"publisher":{"@type":"Organization","name":"Wikimedia Foundation, Inc.","logo":{"@type":"ImageObject","url":"https://www.wikimedia.org/static/images/wmf-hor-googpub.png"}},"datePublished":"2006-08-06T07:34:15Z","dateModified":"2018-12-13T23:44:26Z","image":"https://upload.wikimedia.org/wikipedia/commons/c/c5/Naftidrofuryl.svg","headline":"chemical compound"}(window.RLQ=window.RLQ||).push(function(){mw.config.set({"wgBackendResponseTime":117,"wgHostname":"mw1274"});});

