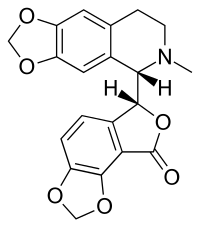
Bicuculline
From Wikipedia, the free encyclopedia
Jump to navigation
Jump to search
Bicuculline
 |
| Clinical data |
|---|
ATC code |
|
|---|
| Identifiers |
|---|
IUPAC name
- (6R)-6-[(5S)-6-methyl-5,6,7,8-tetrahydro[1,3]dioxolo[4,5-g]isoquinolin-5-yl]furo[3,4-e][1,3]benzodioxol-8(6H)-one
|
CAS Number |
485-49-4  Y Y
|
|---|
PubChem CID
|
|
|---|
IUPHAR/BPS |
|
|---|
ChemSpider |
9820  Y Y
|
|---|
UNII |
|
|---|
ChEBI |
CHEBI:3092  N N
|
|---|
ChEMBL |
ChEMBL417990  N N
|
|---|
ECHA InfoCard |
100.006.927 
|
|---|
| Chemical and physical data |
|---|
Formula |
C20H17NO6
|
|---|
Molar mass |
367.352 g/mol |
|---|
3D model (JSmol) |
|
|---|
Melting point |
215 °C (419 °F) |
|---|
SMILES
O=C1O[C@H](c3c1c2OCOc2cc3)[C@@H]5c4cc6OCOc6cc4CCN5C
|
InChI
InChI=1S/C20H17NO6/c1-21-5-4-10-6-14-15(25-8-24-14)7-12(10)17(21)18-11-2-3-13-19(26-9-23-13)16(11)20(22)27-18/h2-3,6-7,17-18H,4-5,8-9H2,1H3/t17-,18+/m0/s1  Y Y
Key:IYGYMKDQCDOMRE-ZWKOTPCHSA-N  Y Y
|
.mw-parser-output .nobold{font-weight:normal}
 N N Y (what is this?) Y (what is this?)
(verify)
|
|---|
Bicuculline is a phthalide-isoquinoline compound that is a light-sensitive competitive antagonist of GABAA receptors. It was originally identified in 1932 in plant alkaloid extracts[1] and has been isolated from Dicentra cucullaria, Adlumia fungosa, and several Corydalis species (all in subfamily Fumarioideae, previously known as family Fumariaceae). Since it blocks the inhibitory action of GABA receptors, the action of bicuculline mimics epilepsy. This property is utilized in laboratories across the world in the in vitro study of epilepsy, generally in hippocampal or cortical neurons in prepared brain slices from rodents. This compound is also routinely used to isolate glutamatergic (excitatory amino acid) receptor function.
The action of bicuculline is primarily on the ionotropic GABAA receptors, which are ligand-gated ion channels concerned chiefly with the passing of chloride ions across the cell membrane, thus promoting an inhibitory influence on the target neuron. These receptors are the major targets for benzodiazepines and related anxiolytic drugs.
The half-maximal inhibitory concentration (IC50) of bicuculline on GABAA receptors is 3 μM.
In addition to being a potent GABAA receptor antagonist, bicuculline can be used to block Ca2+-activated potassium channels.[2]
Sensitivity to bicuculline is defined by IUPHAR as a major criterion in the definition of GABAA receptors
See also[edit]
References[edit]
^ Manske, R. H. F. (1932). "The Alkaloids of Fumaraceous Plants. II. Dicentra cucullaria (L.) Bernh". Canadian Journal of Research. 7 (3): 265–269. doi:10.1139/cjr32-078..mw-parser-output cite.citation{font-style:inherit}.mw-parser-output q{quotes:"""""""'""'"}.mw-parser-output code.cs1-code{color:inherit;background:inherit;border:inherit;padding:inherit}.mw-parser-output .cs1-lock-free a{background:url("//upload.wikimedia.org/wikipedia/commons/thumb/6/65/Lock-green.svg/9px-Lock-green.svg.png")no-repeat;background-position:right .1em center}.mw-parser-output .cs1-lock-limited a,.mw-parser-output .cs1-lock-registration a{background:url("//upload.wikimedia.org/wikipedia/commons/thumb/d/d6/Lock-gray-alt-2.svg/9px-Lock-gray-alt-2.svg.png")no-repeat;background-position:right .1em center}.mw-parser-output .cs1-lock-subscription a{background:url("//upload.wikimedia.org/wikipedia/commons/thumb/a/aa/Lock-red-alt-2.svg/9px-Lock-red-alt-2.svg.png")no-repeat;background-position:right .1em center}.mw-parser-output .cs1-subscription,.mw-parser-output .cs1-registration{color:#555}.mw-parser-output .cs1-subscription span,.mw-parser-output .cs1-registration span{border-bottom:1px dotted;cursor:help}.mw-parser-output .cs1-hidden-error{display:none;font-size:100%}.mw-parser-output .cs1-visible-error{font-size:100%}.mw-parser-output .cs1-subscription,.mw-parser-output .cs1-registration,.mw-parser-output .cs1-format{font-size:95%}.mw-parser-output .cs1-kern-left,.mw-parser-output .cs1-kern-wl-left{padding-left:0.2em}.mw-parser-output .cs1-kern-right,.mw-parser-output .cs1-kern-wl-right{padding-right:0.2em}
^ Khawaled, R.; Bruening-Wright, A.; Adelman, J. P.; Maylie, J. (1999). "Bicuculline Block of Small-conductance Calcium-activated Potassium Channels". Pflügers Archiv : European Journal of Physiology. 438 (3): 314–321. doi:10.1007/s004240050915. ISSN 0031-6768. PMID 10398861.
Opium components
|
|---|
Alkaloids |
- 16-Hydroxythebaine
- Berberine
- Canadine
- Codamine
- Coptisine
- Coreximine
- Cycloartenol
- Cycloartenone
- Cyclolaudenol
- Dehydroreticuline
- Dihydrosanguinarine
- Glaucine
- Isoboldine
- Isocorypalmine
- Laudanidine
- Magnoflorine
- Narceine
- Narceinone
- Norlaudanosoline
- Norsanguinarine
- Oripavine
- Oxysanguinarine
- Palaudine
- Papaverrubine B (O-methyl-porphyroxine)
- Papaverrubine C (epiporphyroxine)
- Reticuline
- Salutaridine (sinoacutine)
- Sanguinarine
- Scoulerine
- Somniferine
- Stepholidine
|
|---|
Morphine group
(Phenanthrenes. Includes opioids) |
- Codeine
- Morphine
- Narcotoline
- Neopine
- Perparin
- Papaverrubine D (porphyroxine)
- Pseudocodeine
- Pseudomorphine
- Thebaine
|
|---|
Isoquinolines |
- Cotarnine
- Eupaverine
- Hydrocotarnine
- Laudanosine
- Laudanine
- Noscapine (narcotine)
- Papaverine
- Papaveraldine
- Xanthaline
|
|---|
Protopine group |
- α-Allocryptopine
- α-Fagarine
- Corycavamine
- Corycavine
- Cryptopine
- Protopine
|
|---|
Tetrahydroprotoberberine group |
- Corydaline
- Corybulbine
- Isocorybulbine
- Capaurine
|
|---|
Aporphine group |
- Dicentrine
- Glaucine
- Corytuberine
- Cularine
- Corydine
- Isocorydine
- Bulbocapnine
|
|---|
Phtalide-isoquinolines |
- Adulmine
- Bicuculline
- Bicucine
- Corlumine
|
|---|
α-Naphthaphenanthridines |
- Chelidonine
- β-Homochelidonoine
- Chelerythrine
- Sanguinarine
|
|---|
Other components |
|
|---|
Neurotoxins
|
|---|
Animal |
- Batrachotoxin
- Bestoxin
- Birtoxin
- Bungarotoxin
- Charybdotoxin
- Conotoxin
- Fasciculin
- Huwentoxin
- Poneratoxin
- Saxitoxin
- Tetrodotoxin
- Vanillotoxin
- Spooky toxin (SsTx)
- Epibatidine
- Zetekitoxin AB
|
|---|
Bacterial |
- Botulinum toxin
- Tetanospasmin
|
|---|
Cyanotoxins |
- Anatoxin-a
- Anatoxin-a(S)
- BMAA
- Saxitoxin
|
|---|
Plant |
- Bicuculline
- Penitrem A
- Picrotoxin
- Strychnine
- Tutin
- Rotenone
- Ginkgotoxin
- Cicutoxin
- Oenanthotoxin
- Thujone
- Volkensin
|
|---|
Mycotoxins |
- Ibotenic acid
- Muscarine
- Muscimol
|
|---|
Pesticides |
- Fenpropathrin
- Tetramethylenedisulfotetramine
- Bromethalin
- Crimidine
- Methamidophos
- Endosulfan
- Fipronil
- Phenylsilatrane
- Chlorophenylsilatrane
- Sulfuryl fluoride
- Mipafox
- Schradan
- Dimefox
|
|---|
Nerve agents |
- Cyclosarin
- EA-3148
- Novichok agent
- Sarin
- Soman
- Tabun
- VE
- VG
- VM
- VP
- VR
- VX
- GV
- EA-3990
- EA-4056
- T-1123
- Octamethylene-bis(5-dimethylcarbamoxyisoquinolinium bromide)
- Fluorotabun
- Chinese VX
- EA-2192
|
|---|
Bicyclic phosphates |
|
|---|
Other |
- Dimethylmercury
- Toxopyrimidine
- IDPN
|
|---|
Convulsants
|
|---|
GABA receptor antagonists |
- Picrotoxin
- Gabazine
- Tetramethylenedisulfotetramine
- Cicutoxin
- Phenylsilatrane
- Tutin
- Pentylenetetrazol
- Thujone
- Endosulfan
- Bicuculline
- Oenanthotoxin
- Fipronil
- EBOB
- BIDN
- Dihydropicrotoxinin
- DMCM
- FG-7142
- Chlorophenylsilatrane
Bicyclic phosphates (TBPS, TBPO, IPTBO)
|
|---|
GABA synthesis inhibitors |
- Allylglycine
- Crimidine
- Ginkgotoxin
- Toxopyrimidine
- Isoniazid
- 3-Mercaptopropionic acid
- 4-Deoxypyridoxine
|
|---|
Glycine receptor antagonists |
- Strychnine
- Tutin
- Picrotoxin
- Dendrobine
|
|---|
Glutamate receptor agonists |
- AMPA
- NMDA
- Kainic acid
- Domoic acid
- Quisqualic acid
- Tetrazolylglycine
|
|---|
Other |
- Methyl fluoroacetate
- Nitrocyclohexane
- Methionine sulfoximine
|
|---|
|
|---|
Ionotropic |
GABAA |
Agonists: (+)-Catechin
- Bamaluzole
Barbiturates (e.g., phenobarbital)
- BL-1020
- DAVA
- Dihydromuscimol
- GABA
- Gabamide
- GABOB
- Gaboxadol (THIP)
- Homotaurine (tramiprosate, 3-APS)
- Ibotenic acid
- iso-THAZ
- iso-THIP
- Isoguvacine
- Isomuscimol
- Isonipecotic acid
- Kojic amine
Lignans (e.g., honokiol)
- Methylglyoxal
- Monastrol
- Muscimol
- Nefiracetam
Neuroactive steroids (e.g., allopregnanolone)
- Org 20599
- PF-6372865
- Phenibut
- Picamilon
- P4S
- Progabide
- Propofol
- Quisqualamine
- SL-75102
- TACA
- TAMP
Terpenoids (e.g., borneol)
- Thiomuscimol
- Tolgabide
- ZAPA
Positive modulators (abridged; see here for a full list): α-EMTBL
Alcohols (e.g., ethanol)
- Anabolic steroids
Avermectins (e.g., ivermectin)
Barbiturates (e.g., phenobarbital)
Benzodiazepines (e.g., diazepam)
Bromide compounds (e.g., potassium bromide)
Carbamates (e.g., meprobamate)
- Carbamazepine
- Chloralose
- Chlormezanone
- Clomethiazole
Dihydroergolines (e.g., ergoloid (dihydroergotoxine))
- Etazepine
- Etifoxine
Fenamates (e.g., mefenamic acid)
Flavonoids (e.g., apigenin, hispidulin)
- Fluoxetine
- Flupirtine
Imidazoles (e.g., etomidate)
Kava constituents (e.g., kavain)
- Lanthanum
- Loreclezole
- Monastrol
Neuroactive steroids (e.g., allopregnanolone, cholesterol, THDOC)
- Niacin
- Nicotinamide (niacinamide)
Nonbenzodiazepines (e.g., β-carbolines (e.g., abecarnil), cyclopyrrolones (e.g., zopiclone), imidazopyridines (e.g., zolpidem), pyrazolopyrimidines (e.g., zaleplon))
- Norfluoxetine
- Petrichloral
Phenols (e.g., propofol)
- Phenytoin
Piperidinediones (e.g., glutethimide)
- Propanidid
Pyrazolopyridines (e.g., etazolate)
Quinazolinones (e.g., methaqualone)
- Retigabine (ezogabine)
- ROD-188
Skullcap constituents (e.g., baicalin)
- Stiripentol
Sulfonylalkanes (e.g., sulfonmethane (sulfonal))
- Topiramate
Valerian constituents (e.g., valerenic acid)
Volatiles/gases (e.g., chloral hydrate, chloroform, diethyl ether, paraldehyde, sevoflurane)
Antagonists: Bicuculline
- Coriamyrtin
- Dihydrosecurinine
- Gabazine (SR-95531)
- Hydrastine
- Hyenachin (mellitoxin)
- PHP-501
- Pitrazepin
- Securinine
- Sinomenine
- SR-42641
- SR-95103
- Thiocolchicoside
- Tutin
Negative modulators: 1,3M1B
- 3M2B
- 11-Ketoprogesterone
- 17-Phenylandrostenol
- α5IA (LS-193,268)
- β-CCB
- β-CCE
- β-CCM
- β-CCP
- β-EMGBL
- Anabolic steroids
- Amiloride
- Anisatin
β-Lactams (e.g., penicillins, cephalosporins, carbapenems)
- Basmisanil
- Bemegride
Bicyclic phosphates (TBPS, TBPO, IPTBO)
- BIDN
- Bilobalide
- Bupropion
- CHEB
- Chlorophenylsilatrane
- Cicutoxin
- Cloflubicyne
- Cyclothiazide
- DHEA
- DHEA-S
- Dieldrin
- (+)-DMBB
- DMCM
- DMPC
- EBOB
- Etbicyphat
- FG-7142 (ZK-31906)
Fiproles (e.g., fipronil)
Flavonoids (e.g., amentoflavone, oroxylin A)
- Flumazenil
Fluoroquinolones (e.g., ciprofloxacin)
- Flurothyl
- Furosemide
- Iomazenil (123I)
- IPTBO
- Isopregnanolone (sepranolone)
- L-655,708
- Laudanosine
- Leptazol
- Lindane
- MaxiPost
- Morphine
- Morphine-3-glucuronide
- MRK-016
- Naloxone
- Naltrexone
- Nicardipine
Nonsteroidal antiandrogens (e.g., apalutamide, bicalutamide, enzalutamide, flutamide, nilutamide)
- Oenanthotoxin
- Pentylenetetrazol (pentetrazol)
- Phenylsilatrane
Picrotoxin (i.e., picrotin, picrotoxinin and dihydropicrotoxinin)
- Pregnenolone sulfate
- Propybicyphat
- PWZ-029
- Radequinil
- Ro 15-4513
- Ro 19-4603
- RO4882224
- RO4938581
- Sarmazenil
- SCS
- Suritozole
- TB-21007
- TBOB
- TBPS
- TCS-1105
- Terbequinil
- TETS
- Thujone
- U-93631
- Zinc
- ZK-93426
|
|---|
GABAA-ρ |
Agonists: BL-1020
- CACA
- CAMP
- Homohypotaurine
- GABA
- GABOB
- Ibotenic acid
- Isoguvacine
- Muscimol
N4-Chloroacetylcytosine arabinoside
- Picamilon
- Progabide
- TACA
- TAMP
- Thiomuscimol
- Tolgabide
Positive modulators: Allopregnanolone
- Alphaxolone
- ATHDOC
- Lanthanides
Antagonists: (S)-2-MeGABA
- (S)-4-ACPBPA
- (S)-4-ACPCA
- 2-MeTACA
- 3-APMPA
- 4-ACPAM
- 4-GBA
cis-3-ACPBPA
- CGP-36742 (SGS-742)
- DAVA
- Gabazine (SR-95531)
- Gaboxadol (THIP)
- I4AA
- Isonipecotic acid
- Loreclezole
- P4MPA
- P4S
- SKF-97541
- SR-95318
- SR-95813
- TPMPA
trans-3-ACPBPA
- ZAPA
Negative modulators: 5α-Dihydroprogesterone
- Bilobalide
- Loreclezole
Picrotoxin (picrotin, picrotoxinin)
- Pregnanolone
- ROD-188
- THDOC
- Zinc
|
|---|
|
|---|
Metabotropic |
GABAB |
Agonists: 1,4-Butanediol
- 4-Fluorophenibut
- Aceburic acid
- Arbaclofen
- Arbaclofen placarbil
- Baclofen
- BL-1020
- GABA
- Gabamide
- GABOB
- GBL
- GHB
- GHBAL
- GHV
- GVL
- Isovaline
- Lesogaberan
- Phenibut
- Picamilon
- Progabide
- Sodium oxybate
- SKF-97,541
- SL 75102
- Tolgabide
- Tolibut
Positive modulators: ADX-71441
- BHF-177
- BHFF
- BSPP
- CGP-7930
- CGP-13501
- GS-39783
- rac-BHFF
- KK-92A
Antagonists: 2-Hydroxysaclofen
- CGP-35348
- CGP-46381
- CGP-52432
- CGP-54626
- CGP-55845
- CGP-64213
- DAVA
- Homotaurine (tramiprosate, 3-APS)
- Phaclofen
- Saclofen
- SCH-50911
- SKF-97541
Negative modulators: Compound 14
|
|---|
|
|---|
- See also
- Receptor/signaling modulators
- GABAA receptor positive modulators
- GABA metabolism/transport modulators
|
Glycine receptor modulators
|
|---|
Receptor
(ligands)
|
GlyR |
Agonists: β-Alanine
- β-ABA (BABA)
- β-AIBA
- Caesium
D-Alanine
D-Serine
- GABA
- Glycine
- Hypotaurine
- Ivermectin
L-Alanine
L-Proline
L-Serine
L-Threonine
- MDL-27531
- Milacemide
- Picolinic acid
- Propofol
- Quisqualamine
- Sarcosine
- Taurine
Positive modulators: Alcohols (e.g., brometone, chlorobutanol (chloretone), ethanol (alcohol), tert-butanol (2M2P), tribromoethanol, trichloroethanol, trifluoroethanol)
- Alkylbenzene sulfonate
- Anandamide
Barbiturates (e.g., pentobarbital, sodium thiopental)
- Chlormethiazole
- D12-116
Dihydropyridines (e.g., nicardipine)
- Etomidate
Ginseng constituents (e.g., ginsenosides (e.g., ginsenoside-Rf))
- Glutamic acid (glutamate)
- Ivermectin
- Ketamine
Neuroactive steroids (e.g., alfaxolone, pregnenolone (eltanolone), pregnenolone acetate, minaxolone, ORG-20599)
- Nitrous oxide
- Penicillin G
- Propofol
- Tamoxifen
- Tetrahydrocannabinol
- Triclofos
Tropeines (e.g., atropine, bemesetron, cocaine, LY-278584, tropisetron, zatosetron)
Volatiles/gases (e.g., chloral hydrate, chloroform, desflurane, diethyl ether (ether), enflurane, halothane, isoflurane, methoxyflurane, sevoflurane, toluene, trichloroethane (methyl chloroform), trichloroethylene)
- Xenon
- Zinc
Antagonists: 2-Aminostrychnine
- 2-Nitrostrychnine
- 4-Phenyl-4-formyl-N-methylpiperidine
- αEMBTL
- Bicuculline
- Brucine
- Cacotheline
- Caffeine
- Colchicine
- Colubrine
- Cyanotriphenylborate
- Dendrobine
- Diaboline
Endocannabinoids (e.g., 2-AG, anandamide (AEA))
- Gaboxadol (THIP)
- Gelsemine
- iso-THAZ
- Isobutyric acid
- Isonipecotic acid
- Isostrychnine
- Laudanosine
- N-Methylbicuculline
- N-Methylstrychnine
- N,N-Dimethylmuscimol
- Nipecotic acid
- Pitrazepin
- Pseudostrychnine
Quinolines (e.g., 4-hydroxyquinoline, 4-hydroxyquinoline-3-carboxylic acid, 5,7-CIQA, 7-CIQ, 7-TFQ, 7-TFQA)
- RU-5135
- Sinomenine
- Strychnine
- Thiocolchicoside
- Tutin
Negative modulators: Amiloride
Benzodiazepines (e.g., bromazepam, clonazepam, diazepam, flunitrazepam, flurazepam)
- Corymine
- Cyanotriphenylborate
- Daidzein
Dihydropyridines (e.g., nicardipine, nifedipine, nitrendipine)
- Furosemide
- Genistein
Ginkgo constituents (e.g., bilobalide, ginkgolides (e.g., ginkgolide A, ginkgolide B, ginkgolide C, ginkgolide J, ginkgolide M))
- Imipramine
- NBQX
Neuroactive steroids (e.g., 3α-androsterone sulfate, 3β-androsterone sulfate, deoxycorticosterone, DHEA sulfate, pregnenolone sulfate, progesterone)
Opioids (e.g., codeine, dextromethorphan, dextrorphan, levomethadone, levorphanol, morphine, oripavine, pethidine, thebaine)
Picrotoxin (i.e., picrotin and picrotoxinin)
- PMBA
- Riluzole
Tropeines (e.g., bemesetron, LY-278584, tropisetron, zatosetron)
- Verapamil
- Zinc
|
|---|
NMDAR |
|
|---|
|
|---|
Transporter
(blockers)
|
GlyT1 |
- ACPPB
- ALX-1393
- ALX-5407 (NFPS)
- AMG-747
- ASP2535
- BIIB-104 (PF-04958242)
- Bitopertin (RG1678/RO4917838)
- CP-802079
- Ethanol (alcohol)
- Glycyldodecylamide
- GSK1018921
- LY-2365109
- ORG-24598
- ORG-25935 (SCH-900435)
- PF-02545920
- PF-03463275
- Sarcosine
- SSR-103,800
- SSR-504,734
|
|---|
GlyT2 |
- Amoxapine
- Ethanol (alcohol)
- NAGly
- Opiranserin (VVZ-149)
- ORG-25543
- VVZ-368
|
|---|
|
|---|
- See also
- Receptor/signaling modulators
- GABA receptor modulators
- GABAA receptor positive modulators
- Ionotropic glutamate receptor modulators
|

Categories:
- Drugs not assigned an ATC code
- GABAA receptor antagonists
- Glycine receptor antagonists
- Convulsants
- Nitrogen heterocycles
- Oxygen heterocycles
- Heterocyclic compounds (3 rings)
Hidden categories:
- Articles with changed EBI identifier
- ECHA InfoCard ID from Wikidata
- Chemical pages without DrugBank identifier
- Articles without KEGG source
- Drugs with no legal status
- Drugboxes which contain changes to verified fields
- Drugboxes which contain changes to watched fields
Navigation menu
Personal tools
- Not logged in
- Talk
- Contributions
- Create account
- Log in
Navigation
- Main page
- Contents
- Featured content
- Current events
- Random article
- Donate to Wikipedia
- Wikipedia store
Interaction
- Help
- About Wikipedia
- Community portal
- Recent changes
- Contact page
Tools
- What links here
- Related changes
- Upload file
- Special pages
- Permanent link
- Page information
- Wikidata item
- Cite this page
Print/export
- Create a book
- Download as PDF
- Printable version
Languages
- تۆرکجه
- Deutsch
- Italiano
- Српски / srpski
- Srpskohrvatski / српскохрватски
- Suomi
Edit links
(window.RLQ=window.RLQ||).push(function(){mw.config.set({"wgPageParseReport":{"limitreport":{"cputime":"0.744","walltime":"0.934","ppvisitednodes":{"value":5060,"limit":1000000},"ppgeneratednodes":{"value":0,"limit":1500000},"postexpandincludesize":{"value":192287,"limit":2097152},"templateargumentsize":{"value":6038,"limit":2097152},"expansiondepth":{"value":16,"limit":40},"expensivefunctioncount":{"value":3,"limit":500},"unstrip-depth":{"value":1,"limit":20},"unstrip-size":{"value":6476,"limit":5000000},"entityaccesscount":{"value":3,"limit":400},"timingprofile":["100.00% 696.384 1 -total"," 71.64% 498.898 1 Template:Drugbox"," 51.56% 359.081 1 Template:Infobox"," 16.36% 113.924 1 Template:Reflist"," 14.49% 100.940 16 Template:Unbulleted_list"," 14.08% 98.023 2 Template:Cite_journal"," 11.20% 78.024 9 Template:Navbox"," 4.95% 34.483 1 Template:Convert"," 4.60% 32.024 1 Template:Infobox_drug/chemical_formula"," 3.49% 24.338 1 Template:GABAergics"]},"scribunto":{"limitreport-timeusage":{"value":"0.256","limit":"10.000"},"limitreport-memusage":{"value":6560825,"limit":52428800}},"cachereport":{"origin":"mw1268","timestamp":"20181229192524","ttl":1900800,"transientcontent":false}}});});{"@context":"https://schema.org","@type":"Article","name":"Bicuculline","url":"https://en.wikipedia.org/wiki/Bicuculline","sameAs":"http://www.wikidata.org/entity/Q3639734","mainEntity":"http://www.wikidata.org/entity/Q3639734","author":{"@type":"Organization","name":"Contributors to Wikimedia projects"},"publisher":{"@type":"Organization","name":"Wikimedia Foundation, Inc.","logo":{"@type":"ImageObject","url":"https://www.wikimedia.org/static/images/wmf-hor-googpub.png"}},"datePublished":"2004-08-16T00:22:21Z","dateModified":"2018-07-24T18:46:42Z","image":"https://upload.wikimedia.org/wikipedia/commons/4/49/Bicuculline.svg","headline":"chemical compound"}(window.RLQ=window.RLQ||).push(function(){mw.config.set({"wgBackendResponseTime":114,"wgHostname":"mw1323"});});


