Phenaglycodol
Phenaglycodol
Jump to navigation
Jump to search
 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Identifiers | |
IUPAC name
| |
| CAS Number |
|
PubChem CID |
|
| ChemSpider |
|
| ECHA InfoCard | 100.001.124 |
| Chemical and physical data | |
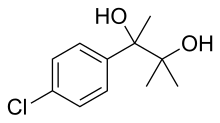
| Formula | C11H15ClO2 |
| Molar mass | 214.689 g/mol |
| 3D model (JSmol) |
|
SMILES
| |
InChI
| |
Phenaglycodol (brand names Acalmid, Acalo, Alterton, Atadiol, Felixyn, Neotran, Pausital, Remin, Sedapsin, Sinforil, Stesil, Ultran)[1] is a drug described as a tranquilizer or sedative which has anxiolytic and anticonvulsant properties.[2][3] It is related structurally and pharmacologically to meprobamate, though it is not a carbamate.[4][5]
Contents
1 Synthesis
1.1 Notes
2 See also
3 References
Synthesis[edit]

Jack Mills "2-chlorophenyl-3-methyl-2, 3-butanediols" U.S. Patent 2,812,363 (1957 to Eli Lilly Co.).
p-Chloroacetophenone and NaCN are reacted together to give the corresponding cyanohydrin (cf Strecker synthesis). The cyano group is then hydrated in acid to the corresponding amide, thus p-chloroatrolactamide (4) is formed. The amide group is then further hydrolyzed with a 2nd equivalent of water in concentrated lye to p-chloroatrolactic acid (5); this is then esterified to Ethyl p-chloroatrolactate (6). Finally, nucleophilic addition a couple of equivalents of MeMgI are added to the ester give Phenaglycodol (7) crystals.
Notes[edit]
- See "Novel trifluoromethyl derivatives of substituted diols" U.S. Patent 3,134,819 also.
See also[edit]
- Metaglycodol
- Fenpentadiol
References[edit]
^ Earl Usdin; Daniel H. Efron; National Institute of Mental Health (U.S.) (1972). Psychotropic drugs and related compounds. National Institute of Mental Health; [for sale by the Supt. of Docs., U.S. Govt. Print. Off., Washington..mw-parser-output cite.citation{font-style:inherit}.mw-parser-output q{quotes:"""""""'""'"}.mw-parser-output code.cs1-code{color:inherit;background:inherit;border:inherit;padding:inherit}.mw-parser-output .cs1-lock-free a{background:url("//upload.wikimedia.org/wikipedia/commons/thumb/6/65/Lock-green.svg/9px-Lock-green.svg.png")no-repeat;background-position:right .1em center}.mw-parser-output .cs1-lock-limited a,.mw-parser-output .cs1-lock-registration a{background:url("//upload.wikimedia.org/wikipedia/commons/thumb/d/d6/Lock-gray-alt-2.svg/9px-Lock-gray-alt-2.svg.png")no-repeat;background-position:right .1em center}.mw-parser-output .cs1-lock-subscription a{background:url("//upload.wikimedia.org/wikipedia/commons/thumb/a/aa/Lock-red-alt-2.svg/9px-Lock-red-alt-2.svg.png")no-repeat;background-position:right .1em center}.mw-parser-output .cs1-subscription,.mw-parser-output .cs1-registration{color:#555}.mw-parser-output .cs1-subscription span,.mw-parser-output .cs1-registration span{border-bottom:1px dotted;cursor:help}.mw-parser-output .cs1-hidden-error{display:none;font-size:100%}.mw-parser-output .cs1-visible-error{font-size:100%}.mw-parser-output .cs1-subscription,.mw-parser-output .cs1-registration,.mw-parser-output .cs1-format{font-size:95%}.mw-parser-output .cs1-kern-left,.mw-parser-output .cs1-kern-wl-left{padding-left:0.2em}.mw-parser-output .cs1-kern-right,.mw-parser-output .cs1-kern-wl-right{padding-right:0.2em}
^ Julius Vida (19 July 2013). Anticonvulsants. Elsevier. pp. 578–. ISBN 978-0-323-14395-0.
^ Lester M. Haddad; James F. Winchester (1983). Clinical Management of Poisoning and Drug Overdose. Saunders. ISBN 978-0-7216-4447-9.
^ Victor Alexander Drill (1958). Pharmacology in Medicine: A Collaborative Textbook. McGraw-Hill.
^ Harry Beckman (1961). Pharmacology; the nature, action and use of drugs. Saunders.
This sedative-related article is a stub. You can help Wikipedia by expanding it. |
Categories:
- Drugs not assigned an ATC code
- Anticonvulsants
- Anxiolytics
- Sedatives
- Sedative stubs
(window.RLQ=window.RLQ||).push(function(){mw.config.set({"wgPageParseReport":{"limitreport":{"cputime":"0.560","walltime":"0.700","ppvisitednodes":{"value":4416,"limit":1000000},"ppgeneratednodes":{"value":0,"limit":1500000},"postexpandincludesize":{"value":122106,"limit":2097152},"templateargumentsize":{"value":3509,"limit":2097152},"expansiondepth":{"value":14,"limit":40},"expensivefunctioncount":{"value":1,"limit":500},"unstrip-depth":{"value":1,"limit":20},"unstrip-size":{"value":13566,"limit":5000000},"entityaccesscount":{"value":1,"limit":400},"timingprofile":["100.00% 551.345 1 -total"," 67.21% 370.565 1 Template:Drugbox"," 47.28% 260.657 1 Template:Infobox"," 18.65% 102.829 1 Template:Reflist"," 15.47% 85.304 5 Template:Cite_book"," 13.82% 76.186 16 Template:Unbulleted_list"," 9.17% 50.563 5 Template:Navbox"," 6.17% 34.001 1 Template:Infobox_drug/chemical_formula"," 5.77% 31.824 1 Template:Anxiolytics"," 4.28% 23.599 1 Template:Infobox_drug/maintenance_categories"]},"scribunto":{"limitreport-timeusage":{"value":"0.190","limit":"10.000"},"limitreport-memusage":{"value":4822730,"limit":52428800}},"cachereport":{"origin":"mw1268","timestamp":"20181222061815","ttl":1900800,"transientcontent":false}}});mw.config.set({"wgBackendResponseTime":86,"wgHostname":"mw1263"});});

