Mianserin
Mianserin
Jump to navigation
Jump to search
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Tolvon, others |
| Synonyms | Mianserin hydrochloride; Org GB 94[1][2] |
| Pregnancy category |
|
| Routes of administration | By mouth |
| ATC code |
|
| Legal status | |
| Legal status |
|
Pharmacokinetic data | |
| Bioavailability | 20–30%[3] |
| Protein binding | 95%[3] |
| Metabolism | Liver (CYP2D6; via aromatic hydroxylation, N-oxidation, N-demethylation)[3] |
| Elimination half-life | 21–61 hours[4] |
| Excretion | Urine: 4–7%[3] Feces: 14–28%[3] |
| Identifiers | |
IUPAC name
| |
| CAS Number |
|
PubChem CID |
|
| IUPHAR/BPS |
|
| DrugBank |
|
| ChemSpider |
|
| UNII |
|
| KEGG |
|
| ChEBI |
|
| ChEMBL |
|
| ECHA InfoCard | 100.041.884 |
| Chemical and physical data | |
| Formula | C18H20N2 |
| Molar mass | 264.365 g/mol |
| 3D model (JSmol) |
|
SMILES
| |
InChI
| |
.mw-parser-output .nobold{font-weight:normal} (verify) | |
Mianserin, sold under the brand name Tolvon among others, is an atypical antidepressant which is used in the treatment of depression in Europe and elsewhere in the world.[5] It is a tetracyclic antidepressant (TeCA). Mianserin is closely related to mirtazapine, both chemically and in terms of its actions and effects, although there are significant differences between the two drugs.
Contents
1 Medical uses
2 Contraindications
3 Side effects
3.1 Withdrawal
4 Overdose
5 Interactions
6 Pharmacology
6.1 Pharmacodynamics
6.2 Pharmacokinetics
7 Chemistry
8 History
9 Society and culture
9.1 Generic names
9.2 Brand names
9.3 Availability
10 Research
11 References
12 Further reading
Medical uses[edit]
Mianserin is used for the treatment of major depressive disorder.[5]
Contraindications[edit]
It should not be given to be people younger than 18 years old, as it can increase the risk of suicide attempts and suicidal thinking, and it can increase aggressiveness.[5]
While there is no evidence that it can harm a fetus from animal models, there is no data showing it safe for pregnant women to take.[5]
People with severe liver disease should not take mianserin, and it should be used with caution for people with epilepsy or who are at risk for seizures, as it can lower the threshold for seizures.[5]
Side effects[edit]
Very common (incidence>10%) adverse effects include constipation, dry mouth, and drowsiness at the beginning of treatment.[4][5]
Common (1%<incidence≤10%) adverse effects include drowsiness during maintenance therapy, tremor, headache, dizziness, vertigo, and weakness.[4]
Uncommon (0.1%<incidence≤1%) adverse effects include weight gain.[4]
Withdrawal[edit]
Abrupt or rapid discontinuation of mianserin may provoke a withdrawal, the effects of which may include depression, anxiety, panic attacks,[6] decreased appetite or anorexia, insomnia, diarrhea, nausea and vomiting, and flu-like symptoms, such as allergies or pruritus, among others.
Overdose[edit]
Overdose of mianserin is known to produce sedation, coma, hypotension or hypertension, tachycardia, and QT interval prolongation.[7]
Interactions[edit]
Mianserin may make drugs that have effects on the brain, like alcohol, anxiolytics, hypnotics and antipsychotics, have stronger effects. It can make antiepileptic medicines work less well. People should not take monoamine oxidase inhibitors and mianserin at the same time.[5]
Carbamazepine and phenobarbital will cause the body to metabolize mianserin faster and may reduce its effects. There is a risk of dangerously low blood pressure if people take mianserin along with diazoxide, hydralazine, or nitroprusside. Mianserin can make antihistamines and antimuscarinics have stronger effects. Mianserin should not be taken with apraclonidine, brimonidine, sibutramine, or the combination drug of artemether with lumefantrine.[5]
Pharmacology[edit]
Pharmacodynamics[edit]
| Site | Ki (nM) | Species | Ref |
|---|---|---|---|
| SERT | 4,000 | Human | [9] |
| NET | 71 | Human | [9] |
| DAT | 9,400 | Human | [9] |
| 5-HT1A | 400–2,600 | Human | [10][11] |
| 5-HT1B | ≥2,800 | Rat | [12] |
| 5-HT1D | 220–400 | Human | [13][14] |
| 5-HT1E | ND | ND | ND |
| 5-HT1F | 13 | Human | [10] |
| 5-HT2A | 1.6–55 | Human | [15][16] |
| 5-HT2B | 1.6–20 | Human | [17][18] |
| 5-HT2C | 0.63–6.5 | Human | [15][19] |
| 5-HT3 | 5.8–300 | Rodent | [20][11] |
| 5-HT4 | ND | ND | ND |
| 5-HT5A | ND | ND | ND |
| 5-HT6 | 55–81 | Human | [21][22] |
| 5-HT7 | 48–56 | Human | [23][24][25] |
| α1 | 34 | Human | [26] |
| α2 | 73 | Human | [26] |
| α2A | 4.8 | Human | [23] |
| α2B | 27 | Human | [27] |
| α2C | 3.8 | Human | [23] |
| D1 | 426–1,420 | Human | [11][23] |
| D2 | 2,100–2,700 | Human | [26][28] |
| D3 | 2,840 | Human | [26] |
| D4 | ND | ND | ND |
| D5 | ND | ND | ND |
| H1 | 0.30–1.7 | Human | [29][26][23] |
| H2 | 437 | Human | [30] |
| H3 | 95,500 | Human | [30] |
| H4 | >100,000 | Human | [30][31] |
| mACh | 820 | Human | [26] |
| MOR | 21,000 | Human | [32] |
| DOR | 30,200 | Human | [32] |
| KOR | 530 (EC50) | Human | [32] |
| Values are Ki (nM), unless otherwise noted. The smaller the value, the more strongly the drug binds to the site. | |||
Mianserin principally exerts its effects via inhibition of histamine, serotonin, acetylcholine and inhibition of norepinephrine reuptake. More specifically, it is an antagonist/inverse agonist at most or all sites of the histamine H1 receptor, serotonin 5-HT1D, 5-HT1F, 5-HT2A, 5-HT2B, 5-HT2C, 5-HT3, 5-HT6, and 5-HT7 receptors, and adrenergic α1- and α2-adrenergic receptors, and additionally a norepinephrine reuptake inhibitor.[33][34] As an H1 receptor inverse agonist with high affinity, mianserin has strong antihistamine effects (e.g., sedation). Conversely, it has low affinity for the muscarinic acetylcholine receptors, and hence lacks anticholinergic properties.[26] Mianserin has been found to be a low affinity but potentially significant partial agonist of the κ-opioid receptor (Ki = 1.7 μM; EC50 = 0.53 μM),[32] similarly to some tricyclic antidepressants (TCAs).[35]
Blockade of the H1 and possibly α1-adrenergic receptors has sedative effects,[4] and also antagonism of the 5-HT2A and α1-adrenergic receptors inhibits activation of intracellular phospholipase C (PLC), which seems to be a common target for several different classes of antidepressants.[36] By antagonizing the somatodendritic and presynaptic α2-adrenergic receptors which function predominantly as inhibitory autoreceptors and heteroreceptors, mianserin disinhibits the release of norepinephrine, dopamine, serotonin, and acetylcholine in various areas of the brain and body.
Along with mirtazapine, although to a lesser extent in comparison, mianserin has sometimes been described as a noradrenergic and specific serotonergic antidepressant (NaSSA).[37] However, the actual evidence in support of this label has been regarded as poor.[38]
Pharmacokinetics[edit]
The bioavailability of mianserin is 20 to 30%.[3] Its plasma protein binding is 95%.[3] Mianserin is metabolized in the liver by the CYP2D6 enzyme via N-oxidation and N-demethylation.[3] Its elimination half-life is 21 to 61 hours.[3] The drug is excreted 4 to 7% in the urine and 14 to 28% in feces.[3]
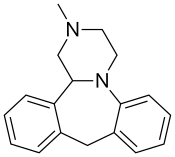
Chemistry[edit]

(S)-Mianserin.
Mianserin is a tetracyclic piperazinoazepine; mirtazapine was developed by the same team of organic chemists and differs via addition of a nitrogen atom in one of the rings.[39][40] (S)-(+)-Mianserin is approximately 200–300 times more active than its enantiomer (R)-(−)-mianserin; hence, the activity of mianserin lies in the (S)-(+) isomer.[citation needed]
History[edit]
It was developed but not discovered by Organon International; the first patents were issued in The Netherlands in 1967, and it was launched in France in 1979 under the brand name Athymil, and soon thereafter in the UK as Norval. Investigators conducting clinical trials in the US submitted fraudulent data, and it was never approved in the US.[41]:21[42]:318
Mianserin was one of the first antidepressants to reach the UK market that was less dangerous than the tricyclic antidepressants in overdose; as of 2012 it was not prescribed much in the UK.[43]
Society and culture[edit]

Mianserin.
Generic names[edit]
Mianserin is the English and German generic name of the drug and its INN and BAN, while mianserin hydrochloride is its USAN, BANM, and JAN. Its generic name in French and its DCF are miansérine, in Spanish and Italian and its DCIT are mianserina, and in Latin is mianserinum.[44][1][45][2]
Brand names[edit]
Mianserin is marketed in many countries mainly under the brand name Tolvon. It is also available throughout the world under a variety of other brand names including Athymil, Bolvidon, Deprevon, Lantanon, Lerivon, Miansan, Serelan, Tetramide, and Tolvin among others.[1][2][44]
Availability[edit]
Mianserin is not approved for use in the United States, but is available in the United Kingdom and other European countries.[46][47] Mianserin received TGA approval in May 1996 and hence is available in Australia.[48]
Research[edit]
The use of mianserin to help people with schizophrenia who are being treated with antipsychotics has been studied in clinical trials; the outcome is unclear.[49][50]
References[edit]
^ abc Index Nominum 2000: International Drug Directory. Taylor & Francis. 2000. pp. 689–. ISBN 978-3-88763-075-1..mw-parser-output cite.citation{font-style:inherit}.mw-parser-output q{quotes:"""""""'""'"}.mw-parser-output code.cs1-code{color:inherit;background:inherit;border:inherit;padding:inherit}.mw-parser-output .cs1-lock-free a{background:url("//upload.wikimedia.org/wikipedia/commons/thumb/6/65/Lock-green.svg/9px-Lock-green.svg.png")no-repeat;background-position:right .1em center}.mw-parser-output .cs1-lock-limited a,.mw-parser-output .cs1-lock-registration a{background:url("//upload.wikimedia.org/wikipedia/commons/thumb/d/d6/Lock-gray-alt-2.svg/9px-Lock-gray-alt-2.svg.png")no-repeat;background-position:right .1em center}.mw-parser-output .cs1-lock-subscription a{background:url("//upload.wikimedia.org/wikipedia/commons/thumb/a/aa/Lock-red-alt-2.svg/9px-Lock-red-alt-2.svg.png")no-repeat;background-position:right .1em center}.mw-parser-output .cs1-subscription,.mw-parser-output .cs1-registration{color:#555}.mw-parser-output .cs1-subscription span,.mw-parser-output .cs1-registration span{border-bottom:1px dotted;cursor:help}.mw-parser-output .cs1-hidden-error{display:none;font-size:100%}.mw-parser-output .cs1-visible-error{font-size:100%}.mw-parser-output .cs1-subscription,.mw-parser-output .cs1-registration,.mw-parser-output .cs1-format{font-size:95%}.mw-parser-output .cs1-kern-left,.mw-parser-output .cs1-kern-wl-left{padding-left:0.2em}.mw-parser-output .cs1-kern-right,.mw-parser-output .cs1-kern-wl-right{padding-right:0.2em}
^ abc "International brands for mianserin". Drugs.com. Retrieved 20 August 2017.
^ abcdefghij Truven Health Analytics, Inc. DRUGDEX® System (Internet) [cited 2013 Sep 29]. Greenwood Village, CO: Thomsen Healthcare; 2013.
^ abcde Merck Sharp & Dohme (Australia) Pty Limited. "Tolvon Product Information" (PDF). GuildLink Pty Ltd.
^ abcdefgh "Mianserin 30 mg film-coated tablets". UK Electronic Medicines Compendium. January 2014. Retrieved 20 August 2017.
^ Kuniyoshi M, Arikawa K, Miura C, Inanaga K (June 1989). "Panic anxiety after abrupt discontinuation of mianserin". Jpn. J. Psychiatry Neurol. 43 (2): 155–9. doi:10.1111/j.1440-1819.1989.tb02564.x. PMID 2796025.
^ Taylor D, Paton C, Kapur S, Taylor D. The Maudsley prescribing guidelines in psychiatry. 11th ed. Chichester, West Sussex: John Wiley & Sons; 2012.
^ Roth, BL; Driscol, J. "PDSP Ki Database". Psychoactive Drug Screening Program (PDSP). University of North Carolina at Chapel Hill and the United States National Institute of Mental Health. Retrieved 14 August 2017.
^ abc Tatsumi M, Groshan K, Blakely RD, Richelson E (1997). "Pharmacological profile of antidepressants and related compounds at human monoamine transporters". Eur. J. Pharmacol. 340 (2–3): 249–58. doi:10.1016/s0014-2999(97)01393-9. PMID 9537821.
^ ab Boess FG, Martin IL (1994). "Molecular biology of 5-HT receptors". Neuropharmacology. 33 (3–4): 275–317. doi:10.1016/0028-3908(94)90059-0. PMID 7984267.
^ abc Toll L, Berzetei-Gurske IP, Polgar WE, et al. (1998). "Standard binding and functional assays related to medications development division testing for potential cocaine and opiate narcotic treatment medications". NIDA Res. Monogr. 178: 440–66. PMID 9686407.
^ Matsumoto I, Combs MR, Jones DJ (1992). "Characterization of 5-hydroxytryptamine1B receptors in rat spinal cord via [125I]iodocyanopindolol binding and inhibition of [3H]-5-hydroxytryptamine release". J. Pharmacol. Exp. Ther. 260 (2): 614–26. PMID 1738111.
^ Peroutka SJ, Switzer JA, Hamik A (1989). "Identification of 5-hydroxytryptamine1D binding sites in human brain membranes". Synapse. 3 (1): 61–6. doi:10.1002/syn.890030109. PMID 2521959.
^ Waeber C, Schoeffter P, Palacios JM, Hoyer D (1988). "Molecular pharmacology of 5-HT1D recognition sites: radioligand binding studies in human, pig and calf brain membranes". Naunyn Schmiedebergs Arch. Pharmacol. 337 (6): 595–601. doi:10.1007/bf00175783. PMID 2975354.
^ ab Millan MJ, Newman-Tancredi A, Audinot V, et al. (2000). "Agonist and antagonist actions of yohimbine as compared to fluparoxan at alpha(2)-adrenergic receptors (AR)s, serotonin (5-HT)(1A), 5-HT(1B), 5-HT(1D) and dopamine D(2) and D(3) receptors. Significance for the modulation of frontocortical monoaminergic transmission and depressive states". Synapse. 35 (2): 79–95. doi:10.1002/(SICI)1098-2396(200002)35:2<79::AID-SYN1>3.0.CO;2-X. PMID 10611634.
^ Elliott JM, Kent A (1989). "Comparison of [125I]iodolysergic acid diethylamide binding in human frontal cortex and platelet tissue". J. Neurochem. 53 (1): 191–6. doi:10.1111/j.1471-4159.1989.tb07313.x. PMID 2723656.
^ Bonhaus DW, Flippin LA, Greenhouse RJ, et al. (1999). "RS-127445: a selective, high affinity, orally bioavailable 5-HT2B receptor antagonist". Br. J. Pharmacol. 127 (5): 1075–82. doi:10.1038/sj.bjp.0702632. PMC 1566110. PMID 10455251.
^ Bonhaus DW, Bach C, DeSouza A, et al. (1995). "The pharmacology and distribution of human 5-hydroxytryptamine2B (5-HT2B) receptor gene products: comparison with 5-HT2A and 5-HT2C receptors". Br. J. Pharmacol. 115 (4): 622–8. doi:10.1111/j.1476-5381.1995.tb14977.x. PMC 1908489. PMID 7582481.
^ Wainscott DB, Lucaites VL, Kursar JD, Baez M, Nelson DL (1996). "Pharmacologic characterization of the human 5-hydroxytryptamine2B receptor: evidence for species differences". J. Pharmacol. Exp. Ther. 276 (2): 720–7. PMID 8632342.
^ Nelson DR, Thomas DR (1989). "[3H]-BRL 43694 (Granisetron), a specific ligand for 5-HT3 binding sites in rat brain cortical membranes". Biochem. Pharmacol. 38 (10): 1693–5. doi:10.1016/0006-2952(89)90319-5. PMID 2543418.
^ Kohen R, Metcalf MA, Khan N, Druck T, Huebner K, Lachowicz JE, Meltzer HY, Sibley DR, Roth BL, Hamblin MW (1996). "Cloning, characterization, and chromosomal localization of a human 5-HT6 serotonin receptor". J. Neurochem. 66 (1): 47–56. doi:10.1046/j.1471-4159.1996.66010047.x. PMID 8522988.
^ Hirst WD, Abrahamsen B, Blaney FE, Calver AR, Aloj L, Price GW, Medhurst AD (2003). "Differences in the central nervous system distribution and pharmacology of the mouse 5-hydroxytryptamine-6 receptor compared with rat and human receptors investigated by radioligand binding, site-directed mutagenesis, and molecular modeling". Mol. Pharmacol. 64 (6): 1295–308. doi:10.1124/mol.64.6.1295. PMID 14645659.
^ abcde Fernández J, Alonso JM, Andrés JI, Cid JM, Díaz A, Iturrino L, Gil P, Megens A, Sipido VK, Trabanco AA (2005). "Discovery of new tetracyclic tetrahydrofuran derivatives as potential broad-spectrum psychotropic agents". J. Med. Chem. 48 (6): 1709–12. doi:10.1021/jm049632c. PMID 15771415.
^ Jasper JR, Kosaka A, To ZP, Chang DJ, Eglen RM (1997). "Cloning, expression and pharmacology of a truncated splice variant of the human 5-HT7 receptor (h5-HT7b)". Br. J. Pharmacol. 122 (1): 126–32. doi:10.1038/sj.bjp.0701336. PMC 1564895. PMID 9298538.
^ Eglen RM, Jasper JR, Chang DJ, Martin GR (1997). "The 5-HT7 receptor: orphan found". Trends Pharmacol. Sci. 18 (4): 104–7. doi:10.1016/s0165-6147(97)01043-2. PMID 9149537.
^ abcdefg Richelson E, Nelson A (1984). "Antagonism by antidepressants of neurotransmitter receptors of normal human brain in vitro". J. Pharmacol. Exp. Ther. 230 (1): 94–102. PMID 6086881.
^ Weinshank RL, Zgombick JM, Macchi M, Adham N, Lichtblau H, Branchek TA, Hartig PR (1990). "Cloning, expression, and pharmacological characterization of a human alpha 2B-adrenergic receptor". Mol. Pharmacol. 38 (5): 681–8. PMID 2172775.
^ Grandy DK, Marchionni MA, Makam H, Stofko RE, Alfano M, Frothingham L, Fischer JB, Burke-Howie KJ, Bunzow JR, Server AC (1989). "Cloning of the cDNA and gene for a human D2 dopamine receptor". Proc. Natl. Acad. Sci. U.S.A. 86 (24): 9762–6. Bibcode:1989PNAS...86.9762G. doi:10.1073/pnas.86.24.9762. PMC 298581. PMID 2532362.
^ Ghoneim OM, Legere JA, Golbraikh A, Tropsha A, Booth RG (2006). "Novel ligands for the human histamine H1 receptor: synthesis, pharmacology, and comparative molecular field analysis studies of 2-dimethylamino-5-(6)-phenyl-1,2,3,4-tetrahydronaphthalenes". Bioorg. Med. Chem. 14 (19): 6640–58. doi:10.1016/j.bmc.2006.05.077. PMID 16782354.
^ abc Appl H, Holzammer T, Dove S, Haen E, Strasser A, Seifert R (2012). "Interactions of recombinant human histamine H₁R, H₂R, H₃R, and H₄R receptors with 34 antidepressants and antipsychotics". Naunyn Schmiedebergs Arch. Pharmacol. 385 (2): 145–70. doi:10.1007/s00210-011-0704-0. PMID 22033803.
^ Nguyen T, Shapiro DA, George SR, Setola V, Lee DK, Cheng R, Rauser L, Lee SP, Lynch KR, Roth BL, O'Dowd BF (2001). "Discovery of a novel member of the histamine receptor family". Mol. Pharmacol. 59 (3): 427–33. PMID 11179435.
^ abcd Olianas MC, Dedoni S, Onali P (November 2012). "The atypical antidepressant mianserin exhibits agonist activity at κ-opioid receptors". Br. J. Pharmacol. 167 (6): 1329–41. doi:10.1111/j.1476-5381.2012.02078.x. PMC 3504997. PMID 22708686.
^ Leonard B, Richelson H (2000). "Synaptic Effects of Antidepressants: Relationship to Their Therapeutic and Adverse Effects". In Buckley JL, Waddington PF. Schizophrenia and Mood Disorders: The New Drug Therapies in Clinical Practice. Oxford: Butterworth-Heinemann. pp. 67–84. ISBN 978-0-7506-4096-1.
^ Müller G (8 May 2006). "Target Family-directed Masterkeys in Chemogenomics". In Kubinyi H, Müller G, Mannhold R, Folkers G. Chemogenomics in Drug Discovery: A Medicinal Chemistry Perspective. John Wiley & Sons. p. 25. ISBN 978-3-527-60402-9.
^ Onali P, Dedoni S, Olianas MC (2010). "Direct agonist activity of tricyclic antidepressants at distinct opioid receptor subtypes". J. Pharmacol. Exp. Ther. 332 (1): 255–65. doi:10.1124/jpet.109.159939. PMID 19828880.
^ Dwivedi Y, Agrawal AK, Rizavi HS, Pandey GN (December 2002). "Antidepressants reduce phosphoinositide-specific phospholipase C (PI-PLC) activity and the mRNA and protein expression of selective PLC beta 1 isozyme in rat brain". Neuropharmacology. 43 (8): 1269–79. doi:10.1016/S0028-3908(02)00253-8. PMID 12527476.
^ Kishi T, Iwata N (2014). "Meta-analysis of noradrenergic and specific serotonergic antidepressant use in schizophrenia". Int. J. Neuropsychopharmacol. 17 (2): 343–54. doi:10.1017/S1461145713000667. PMID 23823741.
^ Gillman PK (2006). "A systematic review of the serotonergic effects of mirtazapine in humans: implications for its dual action status". Hum Psychopharmacol. 21 (2): 117–25. doi:10.1002/hup.750. PMID 16342227.
^ "Mirtazapine label - Australia". GuildLink, a wholly owned subsidiary company of the Pharmacy Guild of Australia. 27 May 2016.
^ Kelder, J; Funke, C; De Boer, T; Delbressine, L; Leysen, D; Nickolson, V (April 1997). "A comparison of the physicochemical and biological properties of mirtazapine and mianserin". The Journal of Pharmacy and Pharmacology. 49 (4): 403–11. doi:10.1111/j.2042-7158.1997.tb06814.x. PMID 9232538.
^ Shorter, Edward (2005). A historical dictionary of psychiatry. Oxford [u.a.]: Oxford Univ. Press. ISBN 978-0-19-517668-1.
^ Stahl, Stephen M. (2013). Stahl's essential psychopharmacology : neuroscientific basis and practical application (4th ed.). Cambridge: Cambridge University Press. ISBN 9781107025981.
^ Pratt, J.P. (2012). "29. Affective Disorders". In Walker, Roger; Whittlesea, Cate. Clinical pharmacy and therapeutics (5th ed.). Edinburgh: Churchill Livingston/Elsevier. p. 472. ISBN 978-0702042935.
^ ab J. Elks (14 November 2014). The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. pp. 822–. ISBN 978-1-4757-2085-3.
^ I.K. Morton; Judith M. Hall (31 October 1999). Concise Dictionary of Pharmacological Agents: Properties and Synonyms. Springer Science & Business Media. pp. 181–. ISBN 978-0-7514-0499-9.
^ Alan J. Gelenberg; Ellen L. Bassuk; Stephen C. Schoonover (29 June 2013). The Practitioner's Guide to Psychoactive Drugs. Springer Science & Business Media. pp. 39–. ISBN 978-1-4757-1137-0.
^ Donald F. Klein; Lewis P. Rowland (24 May 2013). Current Psychotherapeutic Drugs. Routledge. pp. 57–. ISBN 978-1-135-06284-2.
^ AlphaPharm. "Lumin Mianserin hydrochloride product information" (PDF). GuildLink Pty Ltd.
^ Terevnikov, V; Joffe, G; Stenberg, JH (19 May 2015). "Randomized Controlled Trials of Add-On Antidepressants in Schizophrenia". The International Journal of Neuropsychopharmacology. 18 (9): pyv049. doi:10.1093/ijnp/pyv049. PMC 4576515. PMID 25991654.
^ Vernon, JA; Grudnikoff, E; Seidman, AJ; Frazier, TW; Vemulapalli, MS; Pareek, P; Goldberg, TE; Kane, JM; Correll, CU (November 2014). "Antidepressants for cognitive impairment in schizophrenia--a systematic review and meta-analysis". Schizophrenia Research. 159 (2–3): 385–94. doi:10.1016/j.schres.2014.08.015. PMC 4252251. PMID 25240772.
Further reading[edit]
Peet M, Behagel H (1978). "Mianserin: a decade of scientific development". Br. J. Clin. Pharmacol. 5 Suppl 1: 5S–9S. PMC 1429213. PMID 623702.
Categories:
- Alpha-2 blockers
- H1 receptor antagonists
- Kappa agonists
- Noradrenergic and specific serotonergic antidepressants
- Norepinephrine reuptake inhibitors
- Piperazines
- Serotonin antagonists
- Tetracyclic antidepressants
(window.RLQ=window.RLQ||).push(function(){mw.config.set({"wgPageParseReport":{"limitreport":{"cputime":"1.672","walltime":"2.007","ppvisitednodes":{"value":10397,"limit":1000000},"ppgeneratednodes":{"value":0,"limit":1500000},"postexpandincludesize":{"value":744484,"limit":2097152},"templateargumentsize":{"value":183349,"limit":2097152},"expansiondepth":{"value":16,"limit":40},"expensivefunctioncount":{"value":5,"limit":500},"unstrip-depth":{"value":1,"limit":20},"unstrip-size":{"value":155109,"limit":5000000},"entityaccesscount":{"value":4,"limit":400},"timingprofile":["100.00% 1298.930 1 -total"," 40.45% 525.459 1 Template:Drugbox"," 33.03% 429.014 1 Template:Reflist"," 31.42% 408.097 1 Template:Infobox"," 18.09% 235.004 33 Template:Cite_journal"," 12.81% 166.372 28 Template:Navbox"," 8.84% 114.802 16 Template:Unbulleted_list"," 8.83% 114.730 1 Template:Navboxes"," 8.07% 104.837 10 Template:Cite_book"," 6.36% 82.577 60 Template:Abbr"]},"scribunto":{"limitreport-timeusage":{"value":"0.602","limit":"10.000"},"limitreport-memusage":{"value":7207455,"limit":52428800}},"cachereport":{"origin":"mw1341","timestamp":"20181219172802","ttl":1900800,"transientcontent":false}}});mw.config.set({"wgBackendResponseTime":108,"wgHostname":"mw1252"});});

