Benzoctamine
Benzoctamine
Jump to navigation
Jump to search
 | |
| Clinical data | |
|---|---|
| Trade names | Tacitin |
| Routes of administration | Oral, intravenous |
| ATC code |
|
| Legal status | |
| Legal status |
|
Pharmacokinetic data | |
| Bioavailability | 100% for intravenous, 90% for oral |
| Metabolism | Hepatic |
| Elimination half-life | 2 to 3 hours |
| Excretion | Renal |
| Identifiers | |
IUPAC name
| |
| CAS Number |
|
PubChem CID |
|
| DrugBank |
|
| ChemSpider |
|
| UNII |
|
| ChEMBL |
|
| ECHA InfoCard | 100.030.183 |
| Chemical and physical data | |
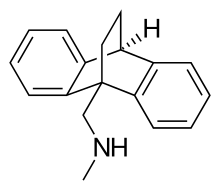
| Formula | C18H19N |
| Molar mass | 249.35 g/mol |
| 3D model (JSmol) |
|
SMILES
| |
InChI
| |
.mw-parser-output .nobold{font-weight:normal} (verify) | |
This article needs to be updated. In particular: Written like it's the 1970s. (April 2017) |
Benzoctamine is a drug that possesses sedative and anxiolytic properties. Marketed as Tacitin by Ciba-Geigy, it is different from most sedative drugs because in most clinical trials it does not produce respiratory depression, but actually stimulates the respiratory system. As a result, when compared to other sedative and anxiolytic drugs such as benzodiazepines like diazepam, it is a safer form of tranquilizing. However, when co-administered with other drugs that cause respiratory depression, like morphine, it can cause increased respiratory depression.
Medically, benzoctamine is used as a treatment for anxious outpatients to control aggression, enuresis, fear, and minor social maladjustment in children. While it is a relatively new anti-anxiety drug, its popularity is increasing as a result of it being able to have comparable anxiolytic and sedative effects to other medications without their potentially fatal respiratory depressive side effects. Its anxiolytic effects are most similar to diazepam, another anxiolytic, but unlike diazepam, benzoctamine has antagonistic effects on epinephrine, norepinephrine, and appears to reduce serotonin turnover. While little is understood about how it carries out its effects, studies point to reduced serotonin, epinephrine, and norepinephrine as partial causes of its pharmacologic and behavioral effects.[1]
Animal studies have shown sedative hypnotic drugs tend to show dependency in animals, but benzoctamine has been shown to not be addictive. Other animal studies also point to the drug as a possible mechanism by which to reduce blood pressure through the adrenergic system.
Chemically, benzoctamine belongs to the class of compounds called dibenzobicyclo-octodienes. It is a tetracyclic compound, consisting of four rings in a three dimensional configuration, and is very closely related structurally to the tetracyclic antidepressant (TeCA) maprotiline, differing only in the length of their side chain.
Contents
1 Medical uses
1.1 Anxiety
1.1.1 Benzoctamine and sodium amylobarbitone
1.1.2 Benzoctamine vs. chlordiazepoxide in anxiety neurosis
1.2 Sleep sedation
1.3 Other uses
1.3.1 Hypertension
2 Side effects
2.1 Common side effects
2.2 Serotonin turnover
3 Pharmacology
4 Pharmacokinetics
5 Other studies
5.1 Alcohol and benzoctamine
5.2 Morphine and benzoctamine
5.3 Dependency
5.4 Benzoctamine vs. chlordiazepoxide in serotonin turnover
6 See also
7 References
Medical uses[edit]
Anxiety[edit]
Benzoctamine’s main clinical use is for the treatment of anxiety, and evidence points to it being as effective as other clinical anxiety drugs, in particular diazepam.[2] In the treatment of symptoms of mild anxiety due to psychoneurosis, a daily dosage of 30 to 80 g of benzoctamine was shown to be just as effective as 6–20 mg of diazepam.[2] In another study one group of patients were given 10g of benzoctamine three times a day, while another group was given 5 mg of diazepam, and the treatments were equivalent.[3] While these studies point to higher doses of benzoctamine being needed to exert the same pharmacological effects, the drug is still popular because of its ability to act as an anxiolytic without producing the common respiratory depression associated with other sedative drugs. Some studies have even shown that it stimulates the respiratory system.[4]
Benzoctamine and sodium amylobarbitone[edit]
In a study used to compare benzoctamine to sodium amylobarbitone as a sleep promoter, it was found that during administration of both drugs, patients reported that their sleep was less restless, and drowsiness was diminished.[5] The study further showed that while sodium amylobarbitone caused withdrawal rebound symptoms, benzoctamine did not.[5] It was also found that benzoctamine reduced plasma corticosteroid hormone levels.[5] There is a relationship between anxiety and adreno-corticosteroid activity, with raised levels commonly being reported as an indication of stress.[5] The study showed that benzoctamine, a drug reported to reduce anxiety, was also able to reduce the hormones that potentially cause it.[5] This points to a phenomenon often seen within pharmacology where drugs intended for other uses often have far-reaching and rarely considered effects.
Benzoctamine vs. chlordiazepoxide in anxiety neurosis[edit]
Benzoctamine has been found to have the same efficacy as chlordiazepoxide when treating anxiety neurosis[6]
Sleep sedation[edit]
While benzoctamine was made to be an alternative to the benzodiazepine line of anxiolytic drugs, other uses for the drug have been discovered. Due to benzoctamine's ability to tranquilize without causing respiratory depression, scientists are moving forward with studies that test its sedative effects in patients with respiratory failure. In one study that used benzoctamine in a clinical setting, researchers showed that the use of benzoctamine for sedation did not result in changes in forced expiratory volume in one second or carbon dioxide partial pressure PCO2.[7] This confirmed previous statements that claimed the drug did not cause respiratory failure. The main goal of this clinical study was to confirm the findings of another study that showed benzoctamine did not reduce CO2 responsiveness, but instead increased the ventilatory response to CO2.[8]
There are usually many risks associated with using sedatives on patients who are suffering from respiratory failure, which has made it difficult to administer tranquillizing medications in situations when they are desirable. It is not known why this drug is safe and its benzodiazepine cousins are not, but a possible explanation for this phenomenon might come from its similarity in structure to tricyclic antidepressants, which have also been shown to not cause respiratory failure.[7] While further experimentation is necessary, this study points to benzoctamine’s possible consideration for sedation in respiratory failure patients.
Other uses[edit]
Hypertension[edit]
A possible treatment for hypertension is blocking peripheral vascular seretonergic neurons or alpha-adrenergic neurons on postsynaptic cell sites.[9] One study showed that benzoctamine, a serotonin and alpha-adrenergic antagonist, does not reduce blood pressure through a seretonin mechanism but does reduce blood pressure by antagonizing alpha-adrenergic receptors in rats.[9] Rats were given 10 mg of benzoctamine and drops in their blood pressure were approximately 30 mm Hg.[9] The researchers further confirmed that serotonin antagonism was not sufficient to reduce blood pressure by using the highly selective serotonin antagonist 1-(1-naphthyl)-piperazine, which was not able to decrease the blood pressure of the rats.[9] These studies have yet to be repeated in humans.
Side effects[edit]
Common side effects[edit]
This section does not cite any sources. (December 2014) (Learn how and when to remove this template message) |
• Drowsiness
• Dry mouth
• Headache
• Dizziness
Serotonin turnover[edit]
Studies have shown that benzoctamine decreases the rate of turnover of serotonin.[9] Scientists confirmed these results and proposed that the method of action was inhibition of serotonin uptake since the drug also blocked the serotonin depleting action of extra-neuronal monoamine transporters (EMT).[10] This would lead to increased stimulation of serotonin receptors through a negative feed back mechanism, eventually decreasing serotonin out put. However, the study points out that other studies have shown that drugs combined with EMT cause a lowering of body temperature that in fact results in a decrease in 5HT turnover.[10] This means that body temperature effects cannot be ruled out.
Pharmacology[edit]
Not much is understood about how benzoctamine produces its anti-anxiety effects, but rat studies have shown that the possible mechanism of action is by way of increased turnover of cathecholamines.[11] In addition to serotonin it has also been shown to decrease epinephrine, dopamine, and norepinephrine turnover by antagonizing their receptors.[10] When given intravenously in doses of 20–40 mg there are no significant differences in efficacy.[12] Oral doses exceeding 10 mg three times daily do not increase the effects of the drug.[3] Assuming serotonin postsynaptic antagonism is the main mechanism by which benzoctamine carries out its effects, studies have shown it to have a half maximal inhibitory concentration(IC50) value of 115 mM at the serotonin receptor.[13]
Pharmacokinetics[edit]
Benzoctamine can be injected directly into the blood or given as tablets. When given as tablets, it is given in doses of 10 mg three times daily.[3] And when given intravenously, patients are given the drug at a rate of 5 mg/minute until 20–40 mg of drug has been injected.[12] Benzoctamine can be analyzed as the 3H acetyl derivative and N-methyl metabolite it gets broken down into using radioactive analysis.[14] Benzoctamine has a half-life of 2–3 hours,[4] with a bioavailability of 100% when given intravenously and around > 90% when given orally.[15] The average time to achieve peak plasma concentrations is 1 hour[4] and the volume of distribution for a 70 kg person is 1-2 l/kg.[4]
Other studies[edit]
Alcohol and benzoctamine[edit]
Benzoctamine, like other psychoactive drugs has the ability to potentiate the effects of other drugs.[16] However, a motor skill study looking at benzoctamine's capacity to potentiate the inhibitory effects of alcohol showed no significant decreases in motor skill function due to benzoctamine being administered with alcohol.[16]
Morphine and benzoctamine[edit]
Though benzoctamine does not potentiate the effects of alcohol, studies have shown it can potentiate the respiratory depression seen with morphine in rats, while also reducing morphine's analgesic effects.[4]
Dependency[edit]
Monkey studies looking at the dependence liability of several sedative drugs showed that benzoctamine was a dependence-free drug, while pentobarbital, alcohol, chloroform, meprobamate, diazepam, chlordiazepoxide, and oxazolam were not.[17]
Benzoctamine vs. chlordiazepoxide in serotonin turnover[edit]
In a rat study looking at the effects of benzoctamine and chlordiazepoxide on serotonin turnover, rats treated with drug were found to have elevated levels of [14C]-5HT, indicating a decrease in serotonin turnover.[1]
See also[edit]
- Maprotiline
References[edit]
^ ab Lippmann W, Pugsley TA; Pugsley (August 1974). "Effects of benzoctamine and chlordiazepoxide on turnover and uptake of 5-hydroxytryptamine in the brain". Br. J. Pharmacol. 51 (4): 571–5. doi:10.1111/j.1476-5381.1974.tb09676.x. PMC 1778070. PMID 4480288..mw-parser-output cite.citation{font-style:inherit}.mw-parser-output q{quotes:"""""""'""'"}.mw-parser-output code.cs1-code{color:inherit;background:inherit;border:inherit;padding:inherit}.mw-parser-output .cs1-lock-free a{background:url("//upload.wikimedia.org/wikipedia/commons/thumb/6/65/Lock-green.svg/9px-Lock-green.svg.png")no-repeat;background-position:right .1em center}.mw-parser-output .cs1-lock-limited a,.mw-parser-output .cs1-lock-registration a{background:url("//upload.wikimedia.org/wikipedia/commons/thumb/d/d6/Lock-gray-alt-2.svg/9px-Lock-gray-alt-2.svg.png")no-repeat;background-position:right .1em center}.mw-parser-output .cs1-lock-subscription a{background:url("//upload.wikimedia.org/wikipedia/commons/thumb/a/aa/Lock-red-alt-2.svg/9px-Lock-red-alt-2.svg.png")no-repeat;background-position:right .1em center}.mw-parser-output .cs1-subscription,.mw-parser-output .cs1-registration{color:#555}.mw-parser-output .cs1-subscription span,.mw-parser-output .cs1-registration span{border-bottom:1px dotted;cursor:help}.mw-parser-output .cs1-hidden-error{display:none;font-size:100%}.mw-parser-output .cs1-visible-error{font-size:100%}.mw-parser-output .cs1-subscription,.mw-parser-output .cs1-registration,.mw-parser-output .cs1-format{font-size:95%}.mw-parser-output .cs1-kern-left,.mw-parser-output .cs1-kern-wl-left{padding-left:0.2em}.mw-parser-output .cs1-kern-right,.mw-parser-output .cs1-kern-wl-right{padding-right:0.2em}
^ ab "Controlled comparative studies with Benzoctamine and Diazepam".
^ abc Goldstein, BJ; Weer, DM (May–Jun 1970). "Comparative evaluation of benzoctamine and diazepam in treatment of anxiety". The Journal of Clinical Pharmacology and the Journal of New Drugs. 10 (3): 194–8. PMID 4392683.
^ abcde Utting, HJ; Pleuvry, BJ (Sep 1975). "Benzoctamine-a study of the respiratory effects of oral doses in human volunteers and interactions with morphine in mice". British Journal of Anaesthesia. 47 (9): 987–92. doi:10.1093/bja/47.9.987. PMID 1103919.
^ abcde Ogunremi, OO; Adamson, L; Brezinová, V; Hunter, WM; Maclean, AW; Oswald, I; Percy-Robb, IW (Apr 28, 1973). "Two anti-anxiety drugs: a psychoneuroendocrine study". British Medical Journal. 2 (5860): 202–5. doi:10.1136/bmj.2.5860.202. PMC 1589389. PMID 4349414.
^ Lo, WH; Lo, T (Jan 1973). "Clinical trial of benzoctamine versus chlordiazepoxide in anxiety neurosis". The Journal of Clinical Pharmacology and New Drugs. 13 (1): 48–53. doi:10.1002/j.1552-4604.1973.tb00069.x. PMID 4566124.
^ ab Clark, TJ; Collins, JV (Jan 13, 1973). "Use of benzoctamine as sedative in patients with respiratory failure". British Medical Journal. 1 (5845): 75–6. doi:10.1136/bmj.1.5845.75. PMC 1588741. PMID 20791872.
^ Geisler, L; Rost, H (1970). "Proceedings of International Symposium on Anxiety and Tension-New Therapeutic Aspects". St. Moritz: 57.
^ abcde Cohen, ML; Fuller, RW; Kurz, KD (Sep–Oct 1983). "Evidence that blood pressure reduction by serotonin antagonists is related to alpha receptor blockade in spontaneously hypertensive rats". Hypertension. 5 (5): 676–81. doi:10.1161/01.hyp.5.5.676. PMID 6311738.
^ abc Lippmann, W; Pugsley, TA (Aug 1974). "Effects of benzoctamine and chlordiazepoxide on turnover and uptake of 5-hydroxytryptamine in the brain". British Journal of Pharmacology. 51 (4): 571–5. doi:10.1111/j.1476-5381.1974.tb09676.x. PMC 1778070. PMID 4480288.
^ Maître, L; Staehelin, M; Bein, HJ (Nov 1970). "Effects of benzoctamine (30803-Ba, TACITIN), a new psychoactive drug, on catecholamine metabolism". Biochemical Pharmacology. 19 (11): 2875–92. doi:10.1016/0006-2952(70)90027-4. PMID 5512696.
^ ab Goodwin, NM; Brock-Utne, JG; Downing, JW; Coleman, AJ (Nov 1974). "Benzoctamine. A preliminary report on a new sedative drug". Anaesthesia. 29 (6): 715–20. doi:10.1111/j.1365-2044.1974.tb00758.x. PMID 4479725.
^ Suzuki-Nishimura, T; Sano, T; Uchida, MK (Aug 11, 1989). "Effects of benzodiazepines on serotonin release from rat mast cells". European Journal of Pharmacology. 167 (1): 75–85. doi:10.1016/0014-2999(89)90749-8. PMID 2550260.
^ Flanagan, RJ (Sep 1995). "The poisoned patient: the role of the laboratory". British journal of biomedical science. 52 (3): 202–13. PMID 8527998.
^ Rang HP, Dale MM, Ritter JM, Moore PK (2003). Pharmacology (5. ed.). Edinburgh [u.a.]: Churchill Livingstone. p. 103. ISBN 0-443-07145-4.
^ ab Landauer, AA; Laurie, W; Milner, G (Aug 1973). "The effect of benzoctamine and alcohol on motor-skills used in car driving". Forensic Science. 2 (3): 275–83. doi:10.1016/0300-9432(73)90042-3. PMID 4740704.
^ Yanagita, T; Takahashi, S (May 1973). "Dependence liability of several sedative-hypnotic agents evaluated in monkeys". The Journal of Pharmacology and Experimental Therapeutics. 185 (2): 307–16. PMID 4634092.
Categories:
- Alpha blockers
- Anxiolytics
- Sedatives
- Serotonin antagonists
- Tetracyclic antidepressants
(window.RLQ=window.RLQ||).push(function(){mw.config.set({"wgPageParseReport":{"limitreport":{"cputime":"1.136","walltime":"1.367","ppvisitednodes":{"value":6338,"limit":1000000},"ppgeneratednodes":{"value":0,"limit":1500000},"postexpandincludesize":{"value":400066,"limit":2097152},"templateargumentsize":{"value":6982,"limit":2097152},"expansiondepth":{"value":16,"limit":40},"expensivefunctioncount":{"value":5,"limit":500},"unstrip-depth":{"value":1,"limit":20},"unstrip-size":{"value":54102,"limit":5000000},"entityaccesscount":{"value":3,"limit":400},"timingprofile":["100.00% 876.599 1 -total"," 47.87% 419.591 1 Template:Drugbox"," 35.12% 307.826 1 Template:Infobox"," 29.52% 258.769 1 Template:Reflist"," 22.56% 197.746 16 Template:Cite_journal"," 18.24% 159.858 20 Template:Navbox"," 11.51% 100.909 16 Template:Unbulleted_list"," 6.38% 55.932 1 Template:Serotonin_receptor_modulators"," 5.93% 51.942 8 Template:Main_other"," 5.30% 46.417 1 Template:Update"]},"scribunto":{"limitreport-timeusage":{"value":"0.348","limit":"10.000"},"limitreport-memusage":{"value":6332306,"limit":52428800}},"cachereport":{"origin":"mw1285","timestamp":"20181221125229","ttl":1900800,"transientcontent":false}}});});{"@context":"https://schema.org","@type":"Article","name":"Benzoctamine","url":"https://en.wikipedia.org/wiki/Benzoctamine","sameAs":"http://www.wikidata.org/entity/Q4890786","mainEntity":"http://www.wikidata.org/entity/Q4890786","author":{"@type":"Organization","name":"Contributors to Wikimedia projects"},"publisher":{"@type":"Organization","name":"Wikimedia Foundation, Inc.","logo":{"@type":"ImageObject","url":"https://www.wikimedia.org/static/images/wmf-hor-googpub.png"}},"datePublished":"2007-08-11T00:56:47Z","dateModified":"2018-10-15T23:58:08Z","image":"https://upload.wikimedia.org/wikipedia/commons/2/21/Benzoctamine.svg","headline":"chemical compound"}(window.RLQ=window.RLQ||).push(function(){mw.config.set({"wgBackendResponseTime":145,"wgHostname":"mw1265"});});

