Palonosetron
Palonosetron
Jump to navigation
Jump to search
 | |
| Clinical data | |
|---|---|
| Pronunciation | /pæləˈnoʊsətrɒn/ pal-ə-NOH-sə-tron |
AHFS/Drugs.com | Monograph |
| MedlinePlus | a610002 |
| License data |
|
| Pregnancy category |
|
| Routes of administration | Intravenous, oral |
| ATC code |
|
| Legal status | |
| Legal status |
|
Pharmacokinetic data | |
| Bioavailability | 97% (oral) |
| Protein binding | 62% |
| Metabolism | Hepatic, 50% (mostly CYP2D6-mediated, CYP3A4 and CYP1A2 also involved) |
| Elimination half-life | Approximately 40–50 hours |
| Excretion | Renal, 80% (of which 49% unchanged); fecal (5 to 8%) |
| Identifiers | |
IUPAC name
| |
| CAS Number |
|
PubChem CID |
|
| IUPHAR/BPS |
|
| DrugBank |
|
| ChemSpider |
|
| UNII |
|
| KEGG |
|
| ChEBI |
|
| ChEMBL |
|
PDB ligand |
|
| ECHA InfoCard | 100.126.539 |
| Chemical and physical data | |
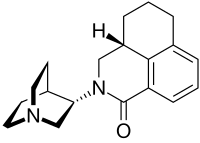
| Formula | C19H24N2O |
| Molar mass | 296.407 g/mol |
| 3D model (JSmol) |
|
| Specific rotation | [α]D −136° [α]D –94.1° (HCl) |
| Melting point | 87 to 88 °C (189 to 190 °F) |
SMILES
| |
InChI
| |
.mw-parser-output .nobold{font-weight:normal} (verify) | |
Palonosetron (INN, trade name Aloxi) is a 5-HT3 antagonist used in the prevention and treatment of chemotherapy-induced nausea and vomiting (CINV). It is used for the control of delayed CINV—nausea and vomiting and there are tentative data to suggest that it may be more effective than granisetron.[1]
Palonosetron is administered intravenously, as a single dose, 30 minutes before chemotherapy,[2] or as a single oral capsule one hour before chemotherapy.[3] It has a longer duration of action than other 5-HT3 antagonists. The oral formulation was approved on August 22, 2008 for prevention of acute CINV alone, as a large clinical trial did not show oral administration to be as effective as intravenous use against delayed CINV.[3]
The oral combination netupitant/palonosetron is approved for both acute and delayed CINV.[4]
Contents
1 Adverse effects
2 Interactions
3 Pharmacology
3.1 Mechanism of action
3.2 Pharmacokinetics
4 Chemistry
5 References
Adverse effects[edit]
The most common adverse effects are headache, which occurs in 4–11% of patients, and constipation in up to 6% of patients. In less than 1% of patients, other gastrointestinal disorders occur, as well as sleeplessness, first- and second-degree atrioventricular block, muscle pain and shortness of breath. Palonosetron is similarly well tolerated as other setrons, and slightly less than placebo.[5][6]
Interactions[edit]
Palonosetron does not relevantly inhibit or induce cytochrome P450 liver enzymes. There are case reports about serotonin syndrome when the drug is combined with serotonergic substances such as selective serotonin reuptake inhibitors (SSRIs) and serotonin–norepinephrine reuptake inhibitors (SNRIs), two common types of antidepressants.[5][6]
Pharmacology[edit]
Mechanism of action[edit]
Palonosetron is a 5-HT3 antagonist, commonly known as a setron. These drugs act by blocking serotonin from binding to the 5-HT3 receptor.[1]
Pharmacokinetics[edit]
Orally taken palonosetron is absorbed well from the gut and has a bioavailability of 97%. Highest blood plasma levels are reached after 5.1±1.7 hours, independently of food intake, and plasma protein binding is 62%. 40% of the substance are eliminated in the unchanged form, and a further 45–50% are metabolized by the liver enzyme CYP2D6 and to a lesser extent by CYP3A4 and CYP1A2. The two main metabolites, the N-oxide and a hydroxy derivative, have less than 1% of palonosetron's antagonistic effect and are thus practically inactive.[5][6]
Palonosetron and its metabolites are mainly (to 80–93%) eliminated via the kidney. Biological half-life in healthy persons was 37±12 hours in a study, and 48±19 hours in cancer patients. In 10% of patients, half-life is over 100 hours.[5][6] Most other marketed setrons have half-lives in the range of about two to 15 hours.

The main metabolites of palonosetron, palonosetron N-oxide (left) and 6S-hydroxy-palonosetron (right)[4]
Chemistry[edit]
The substance is solid at room temperature and melts at 87 to 88 °C (189 to 190 °F).[7] The infusions and capsules contain palonosetron hydrochloride,[5] which is also a solid. The hydrochloride is easily soluble in water, soluble in propylene glycol, and slightly soluble in ethanol and isopropyl alcohol.[6][8]
The molecule has two asymmetric carbon atoms. It is used in form of the pure (S,S)-stereoisomer.[8]
References[edit]
^ ab Billio, A; Morello, E; Clarke, MJ (Jan 20, 2010). "Serotonin receptor antagonists for highly emetogenic chemotherapy in adults". The Cochrane Database of Systematic Reviews (1): CD006272. doi:10.1002/14651858.CD006272.pub2. PMID 20091591..mw-parser-output cite.citation{font-style:inherit}.mw-parser-output q{quotes:"""""""'""'"}.mw-parser-output code.cs1-code{color:inherit;background:inherit;border:inherit;padding:inherit}.mw-parser-output .cs1-lock-free a{background:url("//upload.wikimedia.org/wikipedia/commons/thumb/6/65/Lock-green.svg/9px-Lock-green.svg.png")no-repeat;background-position:right .1em center}.mw-parser-output .cs1-lock-limited a,.mw-parser-output .cs1-lock-registration a{background:url("//upload.wikimedia.org/wikipedia/commons/thumb/d/d6/Lock-gray-alt-2.svg/9px-Lock-gray-alt-2.svg.png")no-repeat;background-position:right .1em center}.mw-parser-output .cs1-lock-subscription a{background:url("//upload.wikimedia.org/wikipedia/commons/thumb/a/aa/Lock-red-alt-2.svg/9px-Lock-red-alt-2.svg.png")no-repeat;background-position:right .1em center}.mw-parser-output .cs1-subscription,.mw-parser-output .cs1-registration{color:#555}.mw-parser-output .cs1-subscription span,.mw-parser-output .cs1-registration span{border-bottom:1px dotted;cursor:help}.mw-parser-output .cs1-hidden-error{display:none;font-size:100%}.mw-parser-output .cs1-visible-error{font-size:100%}.mw-parser-output .cs1-subscription,.mw-parser-output .cs1-registration,.mw-parser-output .cs1-format{font-size:95%}.mw-parser-output .cs1-kern-left,.mw-parser-output .cs1-kern-wl-left{padding-left:0.2em}.mw-parser-output .cs1-kern-right,.mw-parser-output .cs1-kern-wl-right{padding-right:0.2em}
^ De Leon A (2006). "Palonosetron (Aloxi): a second-generation 5-HT(3) receptor antagonist for chemotherapy-induced nausea and vomiting". Proceedings (Baylor University. Medical Center). 19 (4): 413–6. PMC 1618755. PMID 17106506.
^ ab Waknine, Yael (September 4, 2008). "FDA Approvals: Nplate, Aloxi, Vidaza". Medscape. Retrieved 2008-09-04. Freely available with registration.
^ ab "Akynzeo: Summary of Product Characteristics" (PDF). European Medicines Agency. Retrieved 12 July 2016.
^ abcde Haberfeld, H, ed. (2015). Austria-Codex (in German). Vienna: Österreichischer Apothekerverlag.
^ abcde Dinnendahl, V; Fricke, U, eds. (2010). Arzneistoff-Profile (in German). 7 (23 ed.). Eschborn, Germany: Govi Pharmazeutischer Verlag. ISBN 978-3-7741-9846-3.
^ The Merck Index: An Encyclopedia of Chemicals, Drugs, and Biologicals (14 ed.). Merck & Co. 2006. p. 1206. ISBN 978-0-911910-00-1.
^ ab "Chemistry Revire – Aloxi (Palonosetron HCl) Capsules, 0.5 mg" (PDF). Center for Drug Evaluation and Research. 13 August 2008.
Categories:
- 5-HT3 antagonists
- Quinuclidines
- Isoquinolines
- Lactams
(window.RLQ=window.RLQ||).push(function(){mw.config.set({"wgPageParseReport":{"limitreport":{"cputime":"0.856","walltime":"1.091","ppvisitednodes":{"value":5714,"limit":1000000},"ppgeneratednodes":{"value":0,"limit":1500000},"postexpandincludesize":{"value":339354,"limit":2097152},"templateargumentsize":{"value":8655,"limit":2097152},"expansiondepth":{"value":16,"limit":40},"expensivefunctioncount":{"value":3,"limit":500},"unstrip-depth":{"value":1,"limit":20},"unstrip-size":{"value":23739,"limit":5000000},"entityaccesscount":{"value":3,"limit":400},"timingprofile":["100.00% 775.515 1 -total"," 72.22% 560.056 1 Template:Drugbox"," 59.32% 460.035 1 Template:Infobox"," 17.68% 137.081 1 Template:Reflist"," 13.57% 105.226 16 Template:Unbulleted_list"," 12.65% 98.131 18 Template:Navbox"," 10.15% 78.748 2 Template:Cite_journal"," 5.97% 46.302 1 Template:IPAc-en"," 5.94% 46.078 1 Template:Serotonergics"," 5.87% 45.511 1 Template:Infobox_drug/chemical_formula"]},"scribunto":{"limitreport-timeusage":{"value":"0.279","limit":"10.000"},"limitreport-memusage":{"value":7173966,"limit":52428800}},"cachereport":{"origin":"mw1272","timestamp":"20181220215439","ttl":1900800,"transientcontent":false}}});});{"@context":"https://schema.org","@type":"Article","name":"Palonosetron","url":"https://en.wikipedia.org/wiki/Palonosetron","sameAs":"http://www.wikidata.org/entity/Q419841","mainEntity":"http://www.wikidata.org/entity/Q419841","author":{"@type":"Organization","name":"Contributors to Wikimedia projects"},"publisher":{"@type":"Organization","name":"Wikimedia Foundation, Inc.","logo":{"@type":"ImageObject","url":"https://www.wikimedia.org/static/images/wmf-hor-googpub.png"}},"datePublished":"2007-05-16T23:58:25Z","dateModified":"2018-01-06T21:13:47Z","image":"https://upload.wikimedia.org/wikipedia/commons/d/de/Palonosetron_structure.svg","headline":"pharmaceutical drug"}(window.RLQ=window.RLQ||).push(function(){mw.config.set({"wgBackendResponseTime":114,"wgHostname":"mw1241"});});

