Zacopride
Zacopride
Jump to navigation
Jump to search
 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Identifiers | |
IUPAC name
| |
| CAS Number |
|
PubChem CID |
|
| IUPHAR/BPS |
|
| ChemSpider |
|
| UNII |
|
| ChEMBL |
|
| Chemical and physical data | |
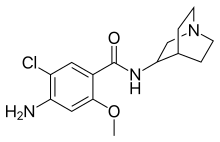
| Formula | C15H20ClN3O2 |
| Molar mass | 309.791 g/mol |
| 3D model (JSmol) |
|
SMILES
| |
InChI
| |
.mw-parser-output .nobold{font-weight:normal} (verify) | |
Zacoprideis a potent antagonist at the 5-HT3 receptor[1] and an agonist at the 5-HT4 receptor.[2] It has anxiolytic[3] and nootropic effects in animal models,[4] with the (R)-(+)-enantiomer being the more active form.[5] It also has antiemetic[6] and pro-respiratory effects, both reducing sleep apnea[7] and reversing opioid-induced respiratory depression in animal studies.[8] Early animal trials have also revealed that administration of zacopride can reduce preference for and consumption of ethanol.[9]
Zacopride was found to significantly increase aldosterone levels in human subjects for 180 minutes at a dose of 400 micrograms. It is thought to do this by stimulating the 5-HT4 receptors on the adrenal glands. Zacopride also stimulated aldosterone secretion when applied to human adrenal glands in vitro. No significant changes were observed in renin, ACTH, or cortisol levels.[2]
Zacopride has been tested in clinical trials for the treatment of schizophrenia, but was found unsuccessful.[10]
References[edit]
^ Smith, WW; Sancilio, LF; Owera-Atepo, JB; Naylor, RJ; Lambert, L (1988). "Zacopride, a potent 5-HT3 antagonist". The Journal of Pharmacy and Pharmacology. 40 (4): 301–2. doi:10.1111/j.2042-7158.1988.tb05253.x. PMID 2900319..mw-parser-output cite.citation{font-style:inherit}.mw-parser-output q{quotes:"""""""'""'"}.mw-parser-output code.cs1-code{color:inherit;background:inherit;border:inherit;padding:inherit}.mw-parser-output .cs1-lock-free a{background:url("//upload.wikimedia.org/wikipedia/commons/thumb/6/65/Lock-green.svg/9px-Lock-green.svg.png")no-repeat;background-position:right .1em center}.mw-parser-output .cs1-lock-limited a,.mw-parser-output .cs1-lock-registration a{background:url("//upload.wikimedia.org/wikipedia/commons/thumb/d/d6/Lock-gray-alt-2.svg/9px-Lock-gray-alt-2.svg.png")no-repeat;background-position:right .1em center}.mw-parser-output .cs1-lock-subscription a{background:url("//upload.wikimedia.org/wikipedia/commons/thumb/a/aa/Lock-red-alt-2.svg/9px-Lock-red-alt-2.svg.png")no-repeat;background-position:right .1em center}.mw-parser-output .cs1-subscription,.mw-parser-output .cs1-registration{color:#555}.mw-parser-output .cs1-subscription span,.mw-parser-output .cs1-registration span{border-bottom:1px dotted;cursor:help}.mw-parser-output .cs1-hidden-error{display:none;font-size:100%}.mw-parser-output .cs1-visible-error{font-size:100%}.mw-parser-output .cs1-subscription,.mw-parser-output .cs1-registration,.mw-parser-output .cs1-format{font-size:95%}.mw-parser-output .cs1-kern-left,.mw-parser-output .cs1-kern-wl-left{padding-left:0.2em}.mw-parser-output .cs1-kern-right,.mw-parser-output .cs1-kern-wl-right{padding-right:0.2em}
^ ab Lefebvre, H; Contesse, V; Delarue, C; Soubrane, C; Legrand, A; Kuhn, JM; Wolf, LM; Vaudry, H (1993). "Effect of the serotonin-4 receptor agonist zacopride on aldosterone secretion from the human adrenal cortex: in vivo and in vitro studies". The Journal of Clinical Endocrinology and Metabolism. 77 (6): 1662–6. doi:10.1210/jc.77.6.1662. PMID 8263156.
^ Costall, B; Domeney, AM; Gerrard, PA; Kelly, ME; Naylor, RJ (1988). "Zacopride: anxiolytic profile in rodent and primate models of anxiety". The Journal of Pharmacy and Pharmacology. 40 (4): 302–5. doi:10.1111/j.2042-7158.1988.tb05254.x. PMID 2900320.
^ Fontana, DJ; Daniels, SE; Eglen, RM; Wong, EH (1996). "Stereoselective effects of (R)- and (S)-zacopride on cognitive performance in a spatial navigation task in rats". Neuropharmacology. 35 (3): 321–7. doi:10.1016/0028-3908(96)00191-8. PMID 8783207.
^ Young, R; Johnson, DN (1991). "Anxiolytic-like activity of R(+)- and S(−)-zacopride in mice". European Journal of Pharmacology. 201 (2–3): 151–5. doi:10.1016/0014-2999(91)90338-Q. PMID 1686755.
^ Yamakuni, H; Nakayama, H; Matsui, S; Imazumi, K; Matsuo, M; Mutoh, S (2006). "Inhibitory effect of zacopride on Cisplatin-induced delayed emesis in ferrets". Journal of pharmacological sciences. 101 (1): 99–102. doi:10.1254/jphs.SCJ05007X. PMID 16651699.
^ Carley, DW; Depoortere, H; Radulovacki, M (2001). "R-zacopride, a 5-HT3 antagonist/5-HT4 agonist, reduces sleep apneas in rats". Pharmacology Biochemistry and Behavior. 69 (1–2): 283–9. doi:10.1016/S0091-3057(01)00535-4. PMID 11420096.
^ Meyer, LC; Fuller, A; Mitchell, D (2006). "Zacopride and 8-OH-DPAT reverse opioid-induced respiratory depression and hypoxia but not catatonic immobilization in goats". American Journal of Physiology. Regulatory, Integrative and Comparative Physiology. 290 (2): R405–13. doi:10.1152/ajpregu.00440.2005. PMID 16166206.
^ Knapp, D.J.; Pohorecky, L.A. (1992). "Zacopride, a 5-HT3 receptor antagonist, reduces voluntary ethanol consumption in rats". Pharmacology Biochemistry and Behavior. 41 (4): 847–850. doi:10.1016/0091-3057(92)90237-A. ISSN 0091-3057.
^ Faustman, William O. (1992). "Zacopride in Schizophrenia: A Single-blind Serotonin Type 3 Antagonist Trial". Archives of General Psychiatry. 49 (9): 751. doi:10.1001/archpsyc.1992.01820090079013. ISSN 0003-990X.
This drug article relating to the nervous system is a stub. You can help Wikipedia by expanding it. |
This drug article relating to the respiratory system is a stub. You can help Wikipedia by expanding it. |
Categories:
- Drugs not assigned an ATC code
- 5-HT3 antagonists
- Anilines
- Benzamides
- Chloroarenes
- Phenol ethers
- Quinuclidines
- Respiratory agents
- Serotonin receptor agonists
- Nervous system drug stubs
- Respiratory system drug stubs
(window.RLQ=window.RLQ||).push(function(){mw.config.set({"wgPageParseReport":{"limitreport":{"cputime":"0.916","walltime":"1.342","ppvisitednodes":{"value":5128,"limit":1000000},"ppgeneratednodes":{"value":0,"limit":1500000},"postexpandincludesize":{"value":313092,"limit":2097152},"templateargumentsize":{"value":4369,"limit":2097152},"expansiondepth":{"value":16,"limit":40},"expensivefunctioncount":{"value":3,"limit":500},"unstrip-depth":{"value":1,"limit":20},"unstrip-size":{"value":30333,"limit":5000000},"entityaccesscount":{"value":3,"limit":400},"timingprofile":["100.00% 1049.455 1 -total"," 45.36% 476.085 1 Template:Drugbox"," 32.80% 344.268 1 Template:Infobox"," 28.22% 296.117 2 Template:Asbox"," 27.98% 293.639 1 Template:Nervous-system-drug-stub"," 17.62% 184.884 1 Template:Reflist"," 15.31% 160.671 10 Template:Cite_journal"," 13.37% 140.336 18 Template:Navbox"," 11.27% 118.254 16 Template:Unbulleted_list"," 6.07% 63.722 1 Template:Serotonergics"]},"scribunto":{"limitreport-timeusage":{"value":"0.317","limit":"10.000"},"limitreport-memusage":{"value":4989769,"limit":52428800}},"cachereport":{"origin":"mw1337","timestamp":"20181215095528","ttl":1900800,"transientcontent":false}}});});{"@context":"https://schema.org","@type":"Article","name":"Zacopride","url":"https://en.wikipedia.org/wiki/Zacopride","sameAs":"http://www.wikidata.org/entity/Q75830","mainEntity":"http://www.wikidata.org/entity/Q75830","author":{"@type":"Organization","name":"Contributors to Wikimedia projects"},"publisher":{"@type":"Organization","name":"Wikimedia Foundation, Inc.","logo":{"@type":"ImageObject","url":"https://www.wikimedia.org/static/images/wmf-hor-googpub.png"}},"datePublished":"2008-07-22T08:54:28Z","dateModified":"2018-11-16T03:04:57Z","image":"https://upload.wikimedia.org/wikipedia/commons/f/f5/Zacopride.svg","headline":"chemical compound"}(window.RLQ=window.RLQ||).push(function(){mw.config.set({"wgBackendResponseTime":119,"wgHostname":"mw1328"});});

