Thujone
Thujone
Jump to navigation
Jump to search
| |||
| Names | |||
|---|---|---|---|
IUPAC names α: (1S,4R,5R)-4-Methyl-1-(propan-2-yl)bicyclo[3.1.0]hexan-3-one β: (1S,4S,5R)-4-methyl-1-propan-2-ylbicyclo[3.1.0]hexan-3-one | |||
| Other names Bicyclo[3.1.0]hexan-3-one, 4-methyl-1-(1-methylethyl)-, [1S-(1α,4α,5α)]- α-Thujone Thujone, cis 3-Thujanone, (1S,4R,5R)-(-)- Thujon 3-Thujanone, (-)- l-Thujone; 4-Methyl-1-(1-methylethyl)bicyclo[3.1.0]hexan-3-one-, (1S,4R,5R)- 3-Thujone; cis-Thujone (Z)-Thujone (-)-Thujone; Bicyclo(3.1.0)hexan-3-one, 4-methyl-1-(1-methylethyl)-, (1S,4R,5R)- NSC 93742 1-isopropyl-4-methylbicyclo[3.1.0]hexan-3-one | |||
| Identifiers | |||
CAS Number |
| ||
3D model (JSmol) |
| ||
ChEBI |
| ||
ChemSpider |
| ||
ECHA InfoCard | 100.008.103 | ||
IUPHAR/BPS |
| ||
PubChem CID |
| ||
InChI
| |||
SMILES
| |||
| Properties | |||
Chemical formula | C10H16O | ||
Molar mass | 152.24 g·mol−1 | ||
Density | 0.92 g/cm3 (β-thujone); 0.9116 g/cm3 (α-thujone) | ||
Melting point | <25 °C | ||
Boiling point | 201 °C (394 °F; 474 K) (β-thujone) | ||
Solubility in water | 407 mg/L | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
Infobox references | |||
Thujone (/ˈθuːdʒoʊn/ (![]() listen)[1]) is a ketone and a monoterpene that occurs naturally in two diastereomeric (epimeric) forms: (−)-α-thujone and (+)-β-thujone.[2][3] It has a menthol odor. Though it is best known as a chemical compound in the spirit absinthe, which contains only small quantities of thujone, it is unlikely to be responsible for absinthe's alleged psychedelic effects. Thujone acts on GABA as an antagonist (opposite to the effects of alcohol) and is also used in perfumery as a component of several essential oils. As a competitive antagonist of GABA, thujone alone is considered to be convulsant,[4] though by interfering with the inhibitory transmitter GABA, it may convey stimulating, mood elevating effects at low doses.
listen)[1]) is a ketone and a monoterpene that occurs naturally in two diastereomeric (epimeric) forms: (−)-α-thujone and (+)-β-thujone.[2][3] It has a menthol odor. Though it is best known as a chemical compound in the spirit absinthe, which contains only small quantities of thujone, it is unlikely to be responsible for absinthe's alleged psychedelic effects. Thujone acts on GABA as an antagonist (opposite to the effects of alcohol) and is also used in perfumery as a component of several essential oils. As a competitive antagonist of GABA, thujone alone is considered to be convulsant,[4] though by interfering with the inhibitory transmitter GABA, it may convey stimulating, mood elevating effects at low doses.
In addition to the naturally occurring (−)-α-thujone and (+)-β-thujone, two other forms are possible: (+)-α-thujone and (−)-β-thujone. In 2016, they were found in nature as well,[5] in Salvia officinalis.
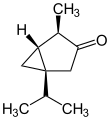
(−)-α-thujone

(+)-α-thujone

(+)-β-thujone

(−)-β-thujone
Contents
1 Sources
2 Biosynthesis
3 Pharmacology
4 In absinthe
4.1 History
5 Regulations
5.1 European Union
5.2 United States
5.3 Canada
6 Chemical Spectra of α-Thujone
6.1 1H NMR (500 MHz, CDCl3)
6.2 13C NMR (91 MHz, CDCl3)
6.3 Mass Spectrometry
6.4 IR
7 See also
8 References
9 Further reading
10 External links
Sources[edit]
Thujone is found in a number of plants, such as arborvitae (genus Thuja, hence the derivation of the name), Nootka cypress, some junipers, mugwort, oregano, common sage, tansy, and wormwood, most notably grand wormwood (Artemisia absinthium), usually as a mix of isomers in a 1:2 ratio. It is also found in various species of Mentha (mint).
Biosynthesis[edit]
The biosynthesis of thujone is similar to the synthesis of other monoterpenes and begins with the formation of geranyl diphosphate (GPP) from Dimethylallyl pyrophosphate (DMAPP) and isopentenyl diphosphate (IPP), catalyzed by the enzyme geranyl diphosphate synthase.[6] Quantitative 13CNMR spectroscopic analysis has demonstrated that the isoprene units used to form thujone in plants are derived from the methylerythritol phosphate pathway (MEP).[7]
The reactions that generate the thujane skeleton in sabinene from GPP are mediated by the enzyme sabinene synthase which has GPP as its substrate.[6]GPP (1) first isomerizes to linalyl diphosphate (LPP) (2) and neryl diphosphate (NPP) (3). LPP preferentially forms a delocalized allylic cation-diphosphate (4). The ion-pair intermediate then cyclizes in an electrophilic addition to yield the α-terpinyl tertiary cation (5).[6]
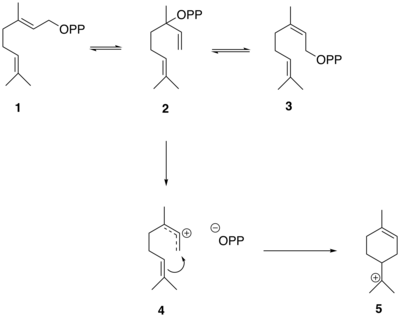
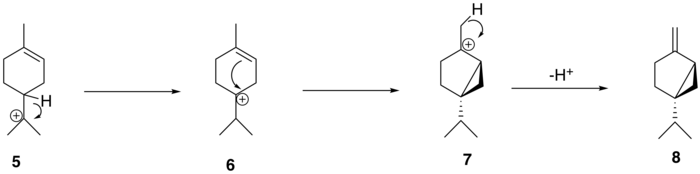
The α-terpinyl cation (5) then undergoes a 1,2 hydride shift via a Wagner–Meerwein rearrangement, leading to the formation of the terpinen-4-yl cation (6). This cation undergoes a second cyclization to form the thujyl cation intermediate (7) before loss of a proton to form the thujone precursor, (+)-sabinene (8).
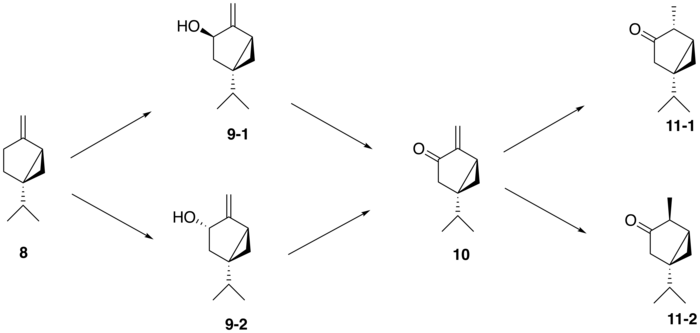
From (+)-sabinene (8), the proposed biosynthetic route to generate thujone follows a three-step pathway: (+)-sabinene is first oxidized to an isomer of (+)-sabinol (9-1,2) by a cytochrome P450 enzyme, followed by conversion to (+)-sabinone (10) via a dehydrogenase. Finally, a reductase mediates the conversion to α-thujone (11-1) and β-thujone (11-2).[8] The isomerism of the (+)-sabinol intermediate varies among thujone-producing plants; for instance, in the western redcedar (Thuja plicata), thujone is derived exclusively from the (+)-trans-sabinol intermediate (9-1) whereas in the common garden sage (Salvia officinalis), thujone is formed from the (+)-cis-sabinol intermediate (9-2).[9]
Pharmacology[edit]

Research-grade thujone
Based on studies that looked only at molecular shape, for many years thujone was thought to act similarly to THC on the cannabinoid receptors;[10] however, this has since been proven false.[11] Thujone is a GABAA receptor antagonist[12] and more specifically, a GABAA receptor competitive antagonist. By inhibiting GABA receptor activation, neurons may fire more easily, which can cause muscle spasms and convulsions.[13] Thujone is also a 5-HT3 antagonist.[14][15]
The median lethal dose, or LD50, of alpha-thujone, the more active of the two isomers, in mice, is around 45 mg/kg, with 0% mortality rate at 30 mg/kg and 100% at 60 mg/kg. Mice exposed to the higher dose have convulsions that lead to death within 1 minute. From 30 to 45 mg/kg, the mice experience muscle spasms in the legs, which progress to general convulsions until death or recovery. These effects are in line with other GABA antagonists. Also, alpha-thujone is metabolized quickly in the liver in mice.[13] Pretreatment with GABA positive allosteric modulators like diazepam, phenobarbital, or 1 g/kg of ethanol protects against a lethal dose of 100 mg/kg.[citation needed]
Attention performance has been tested with low and high doses of thujone in alcohol. The high dose had a short-term negative effect on attention performance. The lower dose showed no noticeable effect.[16]
Thujone is reported[by whom?] to be toxic to brain, kidney, and liver cells and could cause convulsions if used in too high a dose. Other thujone-containing plants such as the tree arborvitae (Thuja occidentalis) are used in herbal medicine, mainly for their immune-system stimulating effects[citation needed]. Side effects from the essential oil of this plant include anxiety, sleeplessness, and convulsions, which confirms the central nervous system effects of thujone.[4][17]
In absinthe[edit]
Thujone is most famous for being a compound in the spirit absinthe. In the past, absinthe was thought to contain up to 260–350 mg/l thujone,[18] but modern tests have shown this estimate to be far too high. A 2008 study of 13 pre-ban (1895–1910) bottles using gas chromatography-mass spectrometry (GC-MS) found that the bottles had between 0.5 and 48.3 mg/l and averaged 25.4 mg/l [19][20] A 2005 study recreated three 1899 high-wormwood recipes and tested with GC-MS, and found that the highest contained 4.3 mg/l thujone.[21] GC-MS testing is important in this capacity, because gas chromatography alone may record an inaccurately high reading of thujone as other compounds may interfere with and add to the apparent measured amount.[22]
History[edit]
The compound was discovered after absinthe became popular in the mid-19th century. Dr. Valentin Magnan, who studied alcoholism, tested pure wormwood oil on animals and discovered it caused seizures independent from the effects of alcohol. Based on this, absinthe, which contains a small amount of wormwood oil, was assumed to be more dangerous than ordinary alcohol. Eventually, thujone was isolated as the cause of these reactions. Magnan went on to study 250 abusers of alcohol and noted that those who drank absinthe had seizures and hallucinations. In light of modern evidence, these conclusions are questionable, as they are based on a poor understanding of other compounds and diseases,[23] and clouded by Magnan's belief that alcohol and absinthe were degenerating the French race.[24]
After absinthe was banned, research dropped off until the 1970s, when the British scientific journal Nature published an article comparing the molecular shape of thujone to tetrahydrocannabinol (THC), the primary psychoactive substance found in cannabis (marijuana), and hypothesized it would act the same way on the brain, sparking the myth that thujone was a cannabinoid.[10][25]
More recently, following European Council Directive No. 88/388/EEC (1988) allowing certain levels of thujone in foodstuffs in the EU,[26] the studies described above were conducted and found only minute levels of thujone in absinthe.
Regulations[edit]
European Union[edit]
Maximum thujone levels in the EU are:[27][28]
- 0.5 mg/kg in food prepared with Artemisia species, excluding those prepared with sage and non alcoholic beverages
- 10 mg/kg in alcoholic beverages not prepared with Artemisia species
- 25 mg/kg in food prepared with sage
- 35 mg/kg in alcoholic beverages prepared with Artemisia species
United States[edit]
In the United States, the addition of pure thujone to foods is not permitted.[29] Foods or beverages that contain Artemisia species, white cedar, oak moss, tansy, or yarrow, must be thujone-free,[30] which in practice means that they contain less than 10 mg/l thujone.[31] Other herbs that contain thujone have no restrictions. For example, sage and sage oil (which can be up to 50% thujone) are on the Food and Drug Administration's list of generally recognized as safe (GRAS) substances.[32]
Absinthe offered for sale in the United States must be thujone-free by the same standard that applies to other beverages containing Artemisia,[31] so absinthe with small amounts of thujone may be legally imported.
Canada[edit]
In Canada, liquor laws are the domain of the provincial governments. Alberta, Ontario, and Nova Scotia allow 10 mg/kg thujone; Quebec allows 15 mg per kg;[citation needed] Manitoba allows 6–8 mg thujone per litre; British Columbia adheres to the same levels as Ontario. However, in Saskatchewan and Quebec, one can purchase any liquor available in the world upon the purchase of a maximum of one case, usually 12 750-ml bottles or 9 L. The individual liquor boards must approve each product before it may be sold on shelves.
Chemical Spectra of α-Thujone[edit]
1H NMR (500 MHz, CDCl3)[edit]
δ [ppm] = 2.54 (ddd, J = 18.8, 2.3, 1.1 Hz, 1H, H-2), 2.21 (q, J = 7.2 Hz, 1H, H-4), 2.07 (d, J = 18.8 Hz, 1H, H-2’), 1.36 (hept, J = 6.8 Hz, 1H, H-7), 1.15 (d, J = 7.5 Hz, 3H, H-9), 1.08 (dd, J = 8.1, 4.0 Hz, 1H, H-5), 1.00 (d, J = 6.8 Hz, 3H, H-8), 0.95 (d, J = 6.8 Hz, 3H, H-8’) 0.76 (ddd, J = 8.1, 5.6, 2.5 Hz, 1H, H-6), 0.12 (dd, J = 5.6, 4.1 Hz, 1H, H-6’).[33]
13C NMR (91 MHz, CDCl3)[edit]
δ [ppm] = 221.7 (C=O, C-3), 47.5 (CH, C-4), 39.9 (CH2, C-2), 33.1 (CH, C-7), 29.8 (C, C-1), 25.7 (CH, C-5), 20.1 (CH3, C-8), 19.9 (CH3, C-8’) 18.9 (CH3, C-9), 18.4 (CH2, C-6).[33]
Mass Spectrometry[edit]
m/z: 81(100), 110(96.58), 109(59.88), 95(58.97), 67(57.37).[34]
IR[edit]
cm-1: 3020, 2961, 1733, 1602, 1455, 1219, 1096, 1014.[35]
See also[edit]
Piołunówka – Polish alcoholic preparation with thujone content higher than in absinthe
References[edit]
^ Derived from the Ancient Greek θυία, thuj(a), a kind of cedar + -ωνη, -one, feminine patronymic for a chemical relative of acetone
^ Perry NB, Anderson RE, Brennan NJ, Douglas MH, Heaney AJ, McGimpsey JA, Smallfield BM (1999). "Essential Oils from Dalmatian Sage (Salvia officinalis L.): Variations among Individuals, Plant Parts, Seasons, and Sites". J. Agric. Food Chem. 47 (5): 2048–2054. doi:10.1021/jf981170m. PMID 10552494..mw-parser-output cite.citation{font-style:inherit}.mw-parser-output q{quotes:"""""""'""'"}.mw-parser-output code.cs1-code{color:inherit;background:inherit;border:inherit;padding:inherit}.mw-parser-output .cs1-lock-free a{background:url("//upload.wikimedia.org/wikipedia/commons/thumb/6/65/Lock-green.svg/9px-Lock-green.svg.png")no-repeat;background-position:right .1em center}.mw-parser-output .cs1-lock-limited a,.mw-parser-output .cs1-lock-registration a{background:url("//upload.wikimedia.org/wikipedia/commons/thumb/d/d6/Lock-gray-alt-2.svg/9px-Lock-gray-alt-2.svg.png")no-repeat;background-position:right .1em center}.mw-parser-output .cs1-lock-subscription a{background:url("//upload.wikimedia.org/wikipedia/commons/thumb/a/aa/Lock-red-alt-2.svg/9px-Lock-red-alt-2.svg.png")no-repeat;background-position:right .1em center}.mw-parser-output .cs1-subscription,.mw-parser-output .cs1-registration{color:#555}.mw-parser-output .cs1-subscription span,.mw-parser-output .cs1-registration span{border-bottom:1px dotted;cursor:help}.mw-parser-output .cs1-hidden-error{display:none;font-size:100%}.mw-parser-output .cs1-visible-error{font-size:100%}.mw-parser-output .cs1-subscription,.mw-parser-output .cs1-registration,.mw-parser-output .cs1-format{font-size:95%}.mw-parser-output .cs1-kern-left,.mw-parser-output .cs1-kern-wl-left{padding-left:0.2em}.mw-parser-output .cs1-kern-right,.mw-parser-output .cs1-kern-wl-right{padding-right:0.2em}
^ Oppolzer W, Pimm A, Stammen B, Hume WE (1997). "Palladium-Catalysed Intramolecular Cyclisations of Olefinic Propargylic Carbonates and application to the diastereoselective synthesis of enantiomerically pure (−)-α-thujone". Helv. Chim. Acta. 80 (3): 623–639. doi:10.1002/hlca.19970800302.
^ ab Olsen, Richard W. (2000-04-25). "Absinthe and γ-aminobutyric acid receptors". Proceedings of the National Academy of Sciences of the United States of America. 97 (9): 4417–4418. ISSN 0027-8424. PMC 34311. PMID 10781032.
^ Williams, Jack D.; Yazarians, Jessica A.; Almeyda, Chelcie C.; Anderson, Kristin A.; Boyce, Gregory R. (23 May 2016). "Detection of the Previously Unobserved Stereoisomers of Thujone in the Essential Oil and Consumable Products of Sage (Salvia officinalis L.) Using Headspace Solid-Phase Microextraction–Gas Chromatography–Mass Spectrometry". Journal of Agricultural and Food Chemistry. 64 (21): 4319–4326. doi:10.1021/acs.jafc.6b01065. PMID 27181395.
^ abc Dewick, Paul M (2009). Medicinal natural products: a biosynthetic approach (3rd ed.). John Wiley & Sons Ltd. pp. 195–197. ISBN 978-0-470-74167-2.
^ Umlauf, Dirk; Zapp, Josef (September 2004). "Biosynthesis of the irregular monoterpene artemisia ketone, the sesquiterpene germacrene D and other isoprenoids in Tanacetum vulgare L. (Asteraceae)". Phytochemistry. 65 (17): 2463–2470. doi:10.1016/j.phytochem.2004.08.019. PMID 15381410.
^ Foster, Adam J.; Hall, Dawn E. (Apr 2013). "Identification of Genes in Thuja plicata Foliar Terpenoid Defenses". Plant Physiology. 161 (4): 1993–2004. doi:10.1104/pp.112.206383. PMC 3613470. PMID 23388118.
^ Gesell, Andreas; Blaukopf, Markus (May 2015). "The Gymnosperm Cytochrome P450 CYP750B1 Catalyzes Stereospecific Monoterpene Hydroxylation of (+)-Sabinene in Thujone Biosynthesis in Western Redcedar". Plant Physiology. 168 (1): 94–106. doi:10.1104/pp.15.00315. PMC 4424034. PMID 25829465.
^ ab Conrad III, Barnaby; (1988). Absinthe: History in a Bottle. Chronicle Books.
ISBN 0-8118-1650-8 p. 152
^ Meschler JP, Howlett AC (March 1999). "Thujone exhibits low affinity for cannabinoid receptors but fails to evoke cannabimimetic responses". Pharmacol. Biochem. Behav. 62 (3): 473–80. doi:10.1016/S0091-3057(98)00195-6. PMID 10080239.
^ Olsen RW (April 2000). "Absinthe and gamma-aminobutyric acid receptors". Proc. Natl. Acad. Sci. U.S.A. 97 (9): 4417–8. doi:10.1073/pnas.97.9.4417. PMC 34311. PMID 10781032.
^ ab Höld KM, Sirisoma NS, Ikeda T, Narahashi T, Casida JE (April 2000). "Alpha-thujone (the active component of absinthe): gamma-aminobutyric acid type A receptor modulation and metabolic detoxification". Proc. Natl. Acad. Sci. U.S.A. 97 (8): 3826–31. doi:10.1073/pnas.070042397. PMC 18101. PMID 10725394.
^ Deiml T, Haseneder R, Zieglgänsberger W, Rammes G, Eisensamer B, Rupprecht R, Hapfelmeier G (Feb 2004). "Alpha-thujone reduces 5-HT3 receptor activity by an effect on the agonist-reduced desensitization". Neuropharmacology. 46 (2): 192–201. doi:10.1016/j.neuropharm.2003.09.022. PMID 15002407.
^ Modulation of Ionotropic GABA Receptors by Natural Products of Plant Origin
^ Dettling A, Grass H, Schuff A, Skopp G, Strohbeck-Kuehner P, Haffner HT (2004). "Absinthe: attention performance and mood under the influence of thujone". J. Stud. Alcohol. 65 (5): 573–81. doi:10.15288/jsa.2004.65.573. PMID 15536765.
^ Naser B, Bodinet C, Tegtmeier M, Lindequist U (Mar 2005). "Thuja occidentalis (Arbor vitae): A Review of its Pharmaceutical, Pharmacological and Clinical Properties". Evidence-Based Complementary and Alternative Medicine. 2 (1): 69–78. doi:10.1093/ecam/neh065. PMC 1062158. PMID 15841280.
^ Absinthism: a fictitious 19th-century syndrome with present impact, Padosch et al. Retrieved Oct. 28, 2006.
^ Absinthe Myths Finally Laid To Rest
^ Chemical Composition of Vintage Preban Absinthe with Special Reference to Thujone, Fenchone, Pinocamphone, Methanol, Copper, and Antimony Concentrations
^ Thujone—Cause of absinthism? Lachenmeier, Emmert et al. Retrieved Oct. 28, 2006.
^ Determination of a-/b-Thujone and Related Terpenes in Absinthe using Solid Phase Extraction and Gas Chromatography, Emmert et al. Retrieved Oct. 28, 2006.
^ Lachenmeier, Dirk; Nathan-Maister, David; Breaux, Theodore; Luaute, Jean-Pierre; Emmert, Joachim (2010). "Absinthe, Absinthism and Thujone – New Insight into the Spirit's Impact on Public Health" (PDF). The Open Addiction Journal. 3: 32–38. doi:10.2174/1874941001003010032. Retrieved 6 July 2016.
^ Conrad III, Barnaby; (1988). Absinthe: History in a Bottle. Chronicle books.
ISBN 0-8118-1650-8 Pg. 101-105
^ Del Castillo J.; Anderson M.; Rubottom G.M. (1975). "Letters to Nature: Marijuana, absinthe and the central nervous system". Nature. 253 (5490): 365–366. doi:10.1038/253365a0.
^ European Council Directive No. 88/388/EEC, 22 June 1988.
^ Regulation (EC) No 1334/2008 of the European Parliament and Council of 16 December 2008, European Commission.
^ Opinion of the Scientific Committee on Food on Thujone Scientific Committee on Food (2003) Retrieved Oct 28, 2006.
^ Laurie C. Dolan; Ray A. Matulka & George A. Burdock (2010). "Naturally Occurring Food Toxins". Toxins. 2 (9): 2289–2332. doi:10.3390/toxins2092289. PMC 3153292. PMID 22069686.
^ FDA Regulation 21 CFR 172.510 – Food Additives Permitted for Direct Addition to Food for Human Consumption. Food and Drug Administration (2003). Retrieved Oct 28, 2006.
^ ab Department of the Treasury Alcohol and Tobacco Tax and Trade Bureau Industry Circular 2007-5 October 17, 2007. Retrieved May 5, 2009
^ Substances generally recognized as safe. Archived 2005-11-30 at the Wayback Machine. Food and Drug Administration (2003). Retrieved Oct 28, 2006.
^ ab Thamm, Irene; Richers, Johannes; Rychlik, Michael; Tiefenbacher, Konrad (2016). "A six-step total synthesis of α-thujone and d6-α-thujone, enabling facile access to isotopically labelled metabolites". Chemical Communications. 52 (78): 11701–11703. doi:10.1039/c6cc05376a. ISSN 1359-7345. PMID 27709180.
^ Movafeghi, A.; Djozan, Dj.; Torbati, S. (2010-08-15). "Solid-phase microextraction of volatile organic compounds released from leaves and flowers ofArtemisia fragrans, followed by GC and GC/MS analysis". Natural Product Research. 24 (13): 1235–1242. doi:10.1080/14786410903108951. ISSN 1478-6419. PMID 20645210.
^ Lightner, David A.; Pak, Chwang Siek; Crist, B.Vincent; Rodgers, Stephen L.; Givens, John W. (January 1985). "The octant rule. XVI1. COnformation and circular dichroism of and 2-methylbicyclo[3.1.0]hexan-3-ones, thujone and isothujone". Tetrahedron. 41 (19): 4321–4330. doi:10.1016/s0040-4020(01)97203-5. ISSN 0040-4020.
.mw-parser-output .refbegin{font-size:90%;margin-bottom:0.5em}.mw-parser-output .refbegin-hanging-indents>ul{list-style-type:none;margin-left:0}.mw-parser-output .refbegin-hanging-indents>ul>li,.mw-parser-output .refbegin-hanging-indents>dl>dd{margin-left:0;padding-left:3.2em;text-indent:-3.2em;list-style:none}.mw-parser-output .refbegin-100{font-size:100%}
Further reading[edit]
Lachenmeier DW, Nathan-Maister D, Breaux TA, Sohnius EM, Schoeberl K, Kuballa T (May 2008). "Chemical composition of vintage preban absinthe with special reference to thujone, fenchone, pinocamphone, methanol, copper, and antimony concentrations". J. Agric. Food Chem. 56 (9): 3073–81. doi:10.1021/jf703568f. PMID 18419128.
External links[edit]
Absinthe absolved, Cern Courier, July 8, 2008
Thujone.Info — Databank of peer reviewed articles on thujone, absinthe, absinthism, and independent thujone ratings of some commercial brands.
The Shaky History of Thujone – Wormwood Society article on thujone and its history.
| Wikimedia Commons has media related to Thujone. |
Categories:
- Absinthe
- GABAA receptor negative allosteric modulators
- Convulsants
- Monoterpenes
- 5-HT3 antagonists
- Ketones
- Perfume ingredients
- Bicyclic compounds
- Cyclopentanes
- Cyclopropanes
- Isopropyl compounds
- Neurotoxins
- Plant toxins
(window.RLQ=window.RLQ||).push(function(){mw.config.set({"wgPageParseReport":{"limitreport":{"cputime":"1.480","walltime":"1.853","ppvisitednodes":{"value":8886,"limit":1000000},"ppgeneratednodes":{"value":0,"limit":1500000},"postexpandincludesize":{"value":457581,"limit":2097152},"templateargumentsize":{"value":30509,"limit":2097152},"expansiondepth":{"value":22,"limit":40},"expensivefunctioncount":{"value":9,"limit":500},"unstrip-depth":{"value":1,"limit":20},"unstrip-size":{"value":80134,"limit":5000000},"entityaccesscount":{"value":4,"limit":400},"timingprofile":["100.00% 1351.504 1 -total"," 41.63% 562.668 1 Template:Chembox"," 26.54% 358.667 1 Template:Reflist"," 23.25% 314.247 1 Template:Chembox_Identifiers"," 20.02% 270.581 20 Template:Cite_journal"," 14.34% 193.862 3 Template:Chembox_headerbar"," 13.86% 187.306 13 Template:Trim"," 12.18% 164.608 22 Template:Navbox"," 9.57% 129.281 1 Template:Chembox_Properties"," 9.18% 124.118 12 Template:Main_other"]},"scribunto":{"limitreport-timeusage":{"value":"0.580","limit":"10.000"},"limitreport-memusage":{"value":10174791,"limit":52428800}},"cachereport":{"origin":"mw1244","timestamp":"20190102102637","ttl":1900800,"transientcontent":false}}});});{"@context":"https://schema.org","@type":"Article","name":"Thujone","url":"https://en.wikipedia.org/wiki/Thujone","sameAs":"http://www.wikidata.org/entity/Q421838","mainEntity":"http://www.wikidata.org/entity/Q421838","author":{"@type":"Organization","name":"Contributors to Wikimedia projects"},"publisher":{"@type":"Organization","name":"Wikimedia Foundation, Inc.","logo":{"@type":"ImageObject","url":"https://www.wikimedia.org/static/images/wmf-hor-googpub.png"}},"datePublished":"2003-01-13T15:24:26Z","dateModified":"2018-12-31T02:51:54Z","image":"https://upload.wikimedia.org/wikipedia/commons/4/46/%28-%29-alpha-Thujon.svg","headline":"chemical compound"}(window.RLQ=window.RLQ||).push(function(){mw.config.set({"wgBackendResponseTime":108,"wgHostname":"mw1258"});});






