2-Bromo-LSD
2-Bromo-LSD
Jump to navigation
Jump to search
 | |
| Clinical data | |
|---|---|
| Synonyms | 2-bromolysergic acid diethylamide, BOL-148 |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
IUPAC name
| |
| CAS Number |
|
PubChem CID |
|
| IUPHAR/BPS |
|
| ChemSpider |
|
| ChEMBL |
|
| Chemical and physical data | |
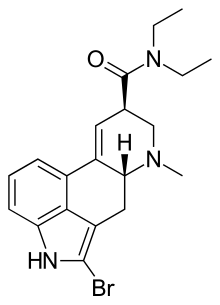
| Formula | C20H24BrN3O |
| Molar mass | 402.328 g·mol−1 |
| 3D model (JSmol) |
|
SMILES
| |
InChI
| |
.mw-parser-output .nobold{font-weight:normal} (verify) | |
2-Bromo-LSD, also known as BOL-148, is a derivative of lysergic acid invented by Albert Hofmann, as part of the original research from which the closely related compound LSD was also derived. 2-bromo-LSD was found to be inactive as a psychedelic and so was comparatively little researched for many years, although its similar behavior in the body made it useful for radiolabelling studies. It was found to bind to many of the same receptor targets as LSD, but acting as a neutral antagonist rather than an agonist. However its generally similar behavior to LSD in some respects has shown to be very useful in one specific area, the treatment of cluster headaches. These debilitating attacks have been known for some time to be amenable to treatment with certain hallucinogenic drugs such as LSD and psilocybin, but because of the illegal status of these drugs and the kind of mental changes they induce, research into their medical use has been slow and therapeutic application limited to very specific circumstances under strict supervision. It had been thought that this specific therapeutic action against cluster headaches was limited to hallucinogenic drugs of this type, and would always present a major barrier to their clinical use. However a serendipitous discovery found that 2-bromo-LSD is also able to produce this therapeutic effect, despite lacking the other effects of LSD. This has led to a resurgence of interest and research into 2-bromo-LSD and its possible medical uses. Some isolated incidents of hallucinogenic responses have been reported, but as with other non-hallucinogenic LSD analogues such as lisuride, this appears to be a rare side effect occurring only in individuals with an as yet unexplained susceptibility to this reaction.[2][3][4][5][6][7][8][9][10]
References[edit]
^ BOL-148 hydrochloride at THC Pharm GmbH
^ Troxler F, Hofmann A. Substitutionen am Ringsystem
der Lysergsäure. I—Substitutionen am Indol-Stickstoff. Helvetica Chimica Acta 1957; 40(VII): 2160–2170.
^ GINZEL KH, MAYER-GROSS W (July 1956). "Prevention of psychological effects of d-lysergic acid diethylamide (LSD 25) by its 2-brom derivative (BOL 148)". Nature. 178 (4526): 210. doi:10.1038/178210a0. PMID 13348662..mw-parser-output cite.citation{font-style:inherit}.mw-parser-output q{quotes:"""""""'""'"}.mw-parser-output code.cs1-code{color:inherit;background:inherit;border:inherit;padding:inherit}.mw-parser-output .cs1-lock-free a{background:url("//upload.wikimedia.org/wikipedia/commons/thumb/6/65/Lock-green.svg/9px-Lock-green.svg.png")no-repeat;background-position:right .1em center}.mw-parser-output .cs1-lock-limited a,.mw-parser-output .cs1-lock-registration a{background:url("//upload.wikimedia.org/wikipedia/commons/thumb/d/d6/Lock-gray-alt-2.svg/9px-Lock-gray-alt-2.svg.png")no-repeat;background-position:right .1em center}.mw-parser-output .cs1-lock-subscription a{background:url("//upload.wikimedia.org/wikipedia/commons/thumb/a/aa/Lock-red-alt-2.svg/9px-Lock-red-alt-2.svg.png")no-repeat;background-position:right .1em center}.mw-parser-output .cs1-subscription,.mw-parser-output .cs1-registration{color:#555}.mw-parser-output .cs1-subscription span,.mw-parser-output .cs1-registration span{border-bottom:1px dotted;cursor:help}.mw-parser-output .cs1-hidden-error{display:none;font-size:100%}.mw-parser-output .cs1-visible-error{font-size:100%}.mw-parser-output .cs1-subscription,.mw-parser-output .cs1-registration,.mw-parser-output .cs1-format{font-size:95%}.mw-parser-output .cs1-kern-left,.mw-parser-output .cs1-kern-wl-left{padding-left:0.2em}.mw-parser-output .cs1-kern-right,.mw-parser-output .cs1-kern-wl-right{padding-right:0.2em}
^ ISBELL H, MINER EJ, LOGAN CR (November 1959). "Cross tolerance between D-2-brom-lysergic acid diethylamide (BOL-148) and the D-diethylamide of lysergic acid (LSD-25)". Psychopharmacologia. 1: 109–16. doi:10.1007/bf00409110. PMID 14405871.
^ King AR, Martin IL, Melville KA (November 1974). "Reversal learning enhanced by lysergic acid diethylamide (LSD): concomitant rise in brain 5-hydroxytryptamine levels". British Journal of Pharmacology. 52 (3): 419–26. doi:10.1111/j.1476-5381.1974.tb08611.x. PMC 1777004. PMID 4458849.
^ Zivin JA, Venditto JA (April 1984). "Experimental CNS ischemia: serotonin antagonists reduce or prevent damage". Neurology. 34 (4): 469–74. doi:10.1212/wnl.34.4.469. PMID 6142430.
^ Harvey JA (2003). "Role of the serotonin 5-HT(2A) receptor in learning". Learning & Memory. 10 (5): 355–62. doi:10.1101/lm.60803. PMC 218001. PMID 14557608.
^ Dave KD, Harvey JA, Aloyo VJ (October 2007). "The time-course for up- and down-regulation of the cortical 5-hydroxytryptamine (5-HT)2A receptor density predicts 5-HT2A receptor-mediated behavior in the rabbit". The Journal of Pharmacology and Experimental Therapeutics. 323 (1): 327–35. doi:10.1124/jpet.107.121707. PMID 17640952.
^ Karst M, Halpern JH, Bernateck M, Passie T (September 2010). "The non-hallucinogen 2-bromo-lysergic acid diethylamide as preventative treatment for cluster headache: an open, non-randomized case series". Cephalalgia: An International Journal of Headache. 30 (9): 1140–4. doi:10.1177/0333102410363490. PMID 20713566.
^ Tfelt-Hansen P (April 2011). "Is BOL-148 hallucinogenic?". Cephalalgia: An International Journal of Headache. 31 (5): 634, author reply 635–6. doi:10.1177/0333102410392069. PMID 21163816.
Categories:
- Drugs not assigned an ATC code
- Antimigraine drugs
- Lysergamides
- Serotonin receptor agonists
(window.RLQ=window.RLQ||).push(function(){mw.config.set({"wgPageParseReport":{"limitreport":{"cputime":"0.808","walltime":"0.961","ppvisitednodes":{"value":4927,"limit":1000000},"ppgeneratednodes":{"value":0,"limit":1500000},"postexpandincludesize":{"value":335478,"limit":2097152},"templateargumentsize":{"value":5046,"limit":2097152},"expansiondepth":{"value":16,"limit":40},"expensivefunctioncount":{"value":3,"limit":500},"unstrip-depth":{"value":1,"limit":20},"unstrip-size":{"value":25544,"limit":5000000},"entityaccesscount":{"value":3,"limit":400},"timingprofile":["100.00% 682.649 1 -total"," 60.88% 415.568 1 Template:Drugbox"," 44.28% 302.285 1 Template:Infobox"," 25.39% 173.354 1 Template:Reflist"," 22.22% 151.707 8 Template:Cite_journal"," 21.44% 146.391 22 Template:Navbox"," 14.39% 98.237 16 Template:Unbulleted_list"," 8.50% 58.041 1 Template:Serotonergics"," 6.90% 47.119 1 Template:Infobox_drug/chemical_formula"," 3.37% 22.976 1 Template:Antimigraine"]},"scribunto":{"limitreport-timeusage":{"value":"0.286","limit":"10.000"},"limitreport-memusage":{"value":4946869,"limit":52428800}},"cachereport":{"origin":"mw1264","timestamp":"20181215085044","ttl":1900800,"transientcontent":false}}});mw.config.set({"wgBackendResponseTime":102,"wgHostname":"mw1327"});});

