Buprenorphine
Buprenorphine
Jump to navigation
Jump to search
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Subutex, others |
AHFS/Drugs.com | Monograph |
| MedlinePlus | a605002 |
| License data |
|
| Pregnancy category |
|
| Routes of administration | Under the tongue, through the cheek, IM, transdermal, intranasal, rectally, by mouth |
| ATC code |
|
| Legal status | |
| Legal status |
|
Pharmacokinetic data | |
| Bioavailability | Sublingual: 30%[1] Intranasal: 48%[2] |
| Protein binding | 96% |
| Metabolism | Liver (CYP3A4, CYP2C8) |
| Onset of action | Within 30 min[3] |
| Elimination half-life | 37 hours (range 20–70 hours) |
| Duration of action | Up to 24 hrs[3] |
| Excretion | Biliary and kidney |
| Identifiers | |
IUPAC name
| |
| CAS Number |
|
PubChem CID |
|
| IUPHAR/BPS |
|
| DrugBank |
|
| ChemSpider |
|
| UNII |
|
| KEGG |
|
| ChEBI |
|
| ChEMBL |
|
| ECHA InfoCard | 100.052.664 |
| Chemical and physical data | |
| Formula | C29H41NO4 |
| Molar mass | 467.64 g/mol |
| 3D model (JSmol) |
|
SMILES
| |
InChI
| |
.mw-parser-output .nobold{font-weight:normal} (verify) | |
Buprenorphine, sold under the brand name Subutex, among others, is an opioid used to treat opioid addiction, acute pain, and chronic pain.[3] It can be used under the tongue, by injection, as a skin patch, or as an implant.[3][4] For opioid addiction it is typically only started when withdrawal symptoms have begun and for the first two days of treatment under direct observation of a health care provider.[3] For longer term treatment of addiction a combination formulation of buprenorphine/naloxone is recommended to prevent misuse by injection.[3] Maximum pain relief is generally within an hour with effects up to 24 hours.[3]
Side effects may include respiratory depression (decreased breathing), sleepiness, adrenal insufficiency, QT prolongation, low blood pressure, allergic reactions, and opioid addiction.[3] Among those with a history of seizures, there is a risk of further seizures.[3]Opioid withdrawal following stopping buprenorphine is generally less severe than with other opioids.[3] It is unclear if use during pregnancy is safe and use while breastfeeding is not recommended.[3] Buprenorphine affects different types of opioid receptors in different ways.[3] Depending on the type of receptor it may be an agonist, partial agonist, or antagonist.[3]
Buprenorphine was approved for medical use in the United States in 1981.[3] In 2012, 9.3 million prescriptions for the medication were written in the United States.[5] Buprenorphine may also be used recreationally by injection or in the nose for the high it produces.[5] Occasionally it is used recreationally instead of heroin.[5] In the United States it is a Schedule III controlled substance.[5] For the tablets the wholesale cost in the United States is between US$0.86 and US$1.32 per daily dose as of 2017.[6]
Contents
1 Medical uses
1.1 Opioid addiction
1.1.1 Buprenorphine versus methadone
1.2 Chronic pain
2 Adverse effects
2.1 Respiratory effects
2.2 Buprenorphine dependence
2.3 Pain management
3 Pharmacology
3.1 Pharmacodynamics
3.1.1 Opioid receptor modulator
3.1.2 Other actions
3.2 Pharmacokinetics
4 Chemistry
4.1 Detection in body fluids
5 History
6 Society and culture
6.1 Regulation
6.2 Brand names
7 Veterinary medicine
8 Research
8.1 Depression
8.2 Cocaine dependence
8.3 Neonatal abstinence
8.4 Obsessive–compulsive disorder
9 References
10 External links
Medical uses[edit]
Opioid addiction[edit]
Its primary use is for the initial treatment of those with opioid addiction.[3] It should only be started once symptoms of withdrawal have begun.[7] For longer term treatment of addiction a combination formulation of buprenorphine/naloxone is usually recommended.[3] A once a month injection, sold under the brandname Sublocade, has been approved in the United States and it should be available in 2018.[8][9]
Buprenorphine versus methadone[edit]
Both buprenorphine and methadone are medications used for detoxification, short- and long-term opioid replacement therapy. Effectiveness of buprenorphine and methadone appear similar, with similar side effects.[10]
Chronic pain[edit]

Butrans patches in the pouch with packaging. A removed patch is shown on the left.
A transdermal patch is available for the treatment of chronic pain.[3] These patches are not indicated for use in acute pain, pain that is expected to last only for a short period of time, or pain after surgery, nor are they recommended for opioid addiction.[11]
Adverse effects[edit]
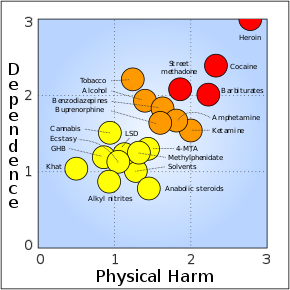
A 2007 assessment of harm from recreational drug use (mean physical harm and mean dependence liability). Buprenorphine was ranked 9th in dependence, 8th in physical harm, and 11th in social harm.[12]
Common adverse drug reactions associated with the use of buprenorphine are similar to those of other opioids and include: nausea and vomiting, drowsiness, dizziness, headache, memory loss, cognitive and neural inhibition, perspiration, itchiness, dry mouth, shrinking of the pupils of the eyes (miosis), orthostatic hypotension, male ejaculatory difficulty, decreased libido, and urinary retention. Constipation and CNS effects are seen less frequently than with morphine.[13]

Allergic contact dermatitis from buprenorphine patch (few days after removal)
Respiratory effects[edit]
The most severe side effect associated with buprenorphine is respiratory depression (insufficient breathing).[3] It occurs more often in those who are also taking benzodiazepines, alcohol, or have underlying lung disease.[3] The usual reversal agents for opioids, such as naloxone, may be only partially effective and additional efforts to support breathing may be required.[3] Respiratory depression may be less than with other opioids, particularly with chronic use.[7] However, in the setting of acute pain management, buprenorphine appears to cause the same rate of respiratory depression as other opioids such as morphine [14]
Buprenorphine dependence[edit]
Buprenorphine treatment carries the risk of causing psychological or physical dependence. Buprenorphine has a slow onset and a long half-life of 24 to 60 hours. Once a person has stabilized on the medication, there are three options: continual use, switching to buprenorphine/naloxone, or medically supervised withdrawal.[7]
Pain management[edit]
It is difficult to achieve acute opioid analgesia in persons using buprenorphine for opioid replacement therapy.[15]
Pharmacology[edit]
Pharmacodynamics[edit]
| Site | Ki (nM) | Action | Species | Ref |
|---|---|---|---|---|
| MOR | 0.21–1.5 0.081 | Partial agonist | Human Monkey | [17][18][19] [20] |
| DOR | 2.9–6.1 0.82 | Antagonist | Human Monkey | [17][19][21] [20] |
| KOR | 0.62–2.5 0.44 | Antagonist | Human Monkey | [17][19][21] [20] |
| NOP | 77.4 | Partial agonist | Human | [18][19][21] |
| σ1 | >100,000 | ND | ND | [22] |
| σ2 | ND | ND | ND | ND |
| NMDA | ND | ND | ND | ND |
| TLR4 | >10,000 | Agonist | Human | [23] |
| SERT | >100,000 | ND | Rat | [24] |
| NET | >100,000 | ND | Rat | [24] |
| DAT | ND | ND | ND | ND |
| VGSC | 33,000 (IC50) | Inhibitor | Rodent | [25] |
| Values are Ki (nM), unless otherwise noted. The smaller the value, the more strongly the drug binds to the site. | ||||
Opioid receptor modulator[edit]
Buprenorphine has been reported to possess the following pharmacological activity:[19]
μ-Opioid receptor (MOR): Partial agonist. Binds with high affinity, but only partially activates the receptor. This property allows buprenorphine to act similarly to full opioid agonists at lower doses (mainly in non-tolerant individuals), reaching a ceiling/plateau at higher doses after which there is no further increase in typical opioid effects (theraputic or recreational).[26] This behavior is responsible for buprenorphine's ability to block most MOR agonists and the phenomenon of withdrawal effects when used in actively opioid dependent persons.
κ-Opioid receptor (KOR): Antagonist.[27]
δ-Opioid receptor (DOR): Antagonist.[27]
Nociceptin receptor (NOP, ORL-1): Weak affinity. Very weak partial agonist. May be involved in lack of respiratory depression with buprenorphine in overdose.
In simplified terms, buprenorphine can essentially be thought of as a non-selective, mixed agonist–antagonist opioid receptor modulator,[28] acting as a weak partial agonist of the MOR, an antagonist of the KOR, an antagonist of the DOR, and a relatively low-affinity, very weak partial agonist of the ORL-1.[21][29][30][31][32][33]
Although buprenorphine is a partial agonist of the MOR, human studies have found that it acts like a full agonist with respect to analgesia in non-opioid-tolerant individuals.[34] Conversely, buprenorphine behaves like a partial agonist of the MOR with respect to respiratory depression.[34]
Buprenorphine is also known to bind to with high affinity and antagonize the putative ε-opioid receptor.[35][36]
Full analgesic efficacy of buprenorphine requires both exon 11-[37] and exon 1-associated μ-opioid receptor splice variants.[38]
The active metabolites of buprenorphine are not thought to be clinically important in its central nervous system effects.[34]
Other actions[edit]
Unlike some other opioids and opioid antagonists, buprenorphine binds only weakly to and possesses little if any activity at the sigma receptor.[39][40]
Buprenorphine also blocks voltage-gated sodium channels via the local anesthetic binding site, and this underlies its potent local anesthetic properties.[25]
Similarly to various other opioids, buprenorphine has also been found to act as an agonist of the toll-like receptor 4, albeit with very low affinity.[23]
Pharmacokinetics[edit]
Buprenorphine is metabolized by the liver, via CYP3A4 (also CYP2C8 seems to be involved) isozymes of the cytochrome P450 enzyme system, into norbuprenorphine (by N-dealkylation). The glucuronidation of buprenorphine is primarily carried out by UGT1A1 and UGT2B7, and that of norbuprenorphine by UGT1A1 and UGT1A3. These glucuronides are then eliminated mainly through excretion into bile. The elimination half-life of buprenorphine is 20 to 73 hours (mean 37 hours). Due to the mainly hepatic elimination, there is no risk of accumulation in people with renal impairment.[41]
One of the major active metabolites of buprenorphine is norbuprenorphine, which, in contrast to buprenorphine itself, is a full agonist of the MOR, DOR, and ORL-1, and a partial agonist at the KOR.[42][43] However, relative to buprenorphine, norbuprenorphine has extremely little antinociceptive potency (1/50th that of buprenorphine), but markedly depresses respiration (10-fold more than buprenorphine).[44] This may be explained by very poor brain penetration of norbuprenorphine due to a high affinity of the compound for P-glycoprotein.[44] In contrast to norbuprenorphine, buprenorphine and its glucuronide metabolites are negligibly transported by P-glycoprotein.[44]
The glucuronides of buprenorphine and norbuprenorphine are also biologically active, and represent major active metabolites of buprenorphine.[45]Buprenorphine-3-glucuronide has affinity for the MOR (Ki = 4.9 pM), DOR (Ki = 270 nM) and ORL-1 (Ki = 36 µM), and no affinity for the KOR. It has a small antinociceptive effect and no effect on respiration. Norbuprenorphine-3-glucuronide has no affinity for the MOR or DOR, but does bind to the KOR (Ki = 300 nM) and ORL-1 (Ki = 18 µM). It has a sedative effect but no effect on respiration.
Chemistry[edit]
Buprenorphine is a semi-synthetic analogue of thebaine[46] and is fairly soluble in water, as its hydrochloride salt.[47] It degrades in the presence of light.[47]
Detection in body fluids[edit]
Buprenorphine and norbuprenorphine may be quantitated in blood or urine to monitor use or abuse, confirm a diagnosis of poisoning, or assist in a medicolegal investigation. There is a significant overlap of drug concentrations in body fluids within the possible spectrum of physiological reactions ranging from asymptomatic to comatose. Therefore, it is critical to have knowledge of both the route of administration of the drug and the level of tolerance to opioids of the individual when results are interpreted.[48]
History[edit]
In 1969, researchers at Reckitt & Colman (now Reckitt Benckiser) had spent 10 years attempting to synthesize an opioid compound "with structures substantially more complex than morphine [that] could retain the desirable actions whilst shedding the undesirable side effects". Physical dependence and withdrawal from buprenorphine itself remain important issues since buprenorphine is a long-acting opioid.[49] Reckitt found success when researchers synthesized RX6029 which had showed success in reducing dependence in test animals. RX6029 was named buprenorphine and began trials on humans in 1971.[50][51] By 1978, buprenorphine was first launched in the UK as an injection to treat severe pain, with a sublingual formulation released in 1982.
Society and culture[edit]
Regulation[edit]
In the United States, buprenorphine (Subutex) and buprenorphine with naloxone (Suboxone) were approved for opioid addiction by the United States Food and Drug Administration in October 2002.[52] The FDA rescheduled buprenorphine from a Schedule V drug to a Schedule III drug just before approval of Subutex and Suboxone. The ACSCN for buprenorphine is 9064, and being a Schedule III substance it does not have an annual manufacturing quota imposed by the DEA.[53] The salt in use is the hydrochloride, which has a free base conversion ratio of 0.928.
In the years prior to Suboxone's approval, Reckitt Benckiser had lobbied Congress to help craft the Drug Addiction Treatment Act of 2000 (DATA 2000), which gave authority to the Secretary of Health and Human Services to grant a waiver to physicians with certain training to prescribe and administer Schedule III, IV, or V narcotic drugs for the treatment of addiction or detoxification. Prior to the passage of this law, such treatment was not permitted in outpatient settings except for clinics designed specifically for drug addiction.[54]
The waiver, which can be granted after the completion of an eight-hour course, is required for outpatient treatment of opioid addiction with Subutex and Suboxone. Initially, the number of patients each approved physician could treat was limited to ten. This was eventually modified to allow approved physicians to treat up to a hundred patients with buprenorphine for opioid addiction in an outpatient setting.[55] This limit was recently increased by the Obama administration, raising the number of patients to which doctors can prescribe to 275.[56] Still, due to this patient limit and the requisite eight-hour training course, many continuing patients can find it very difficult to get a prescription, despite the drug's effectiveness.[57]
In the European Union, Subutex and Suboxone, buprenorphine's high-dose sublingual tablet preparations, were approved for opioid addiction treatment in September 2006.[58] In the Netherlands, buprenorphine is a List II drug of the Opium Law, though special rules and guidelines apply to its prescription and dispensation.
Brand names[edit]
Buprenorphine is available under the trade names Cizdol, Suboxone, Subutex (typically used for opioid addiction), Temgesic (sublingual tablets for moderate to severe pain), Buprenex (solutions for injection often used for acute pain in primary-care settings), Norspan and Butrans (transdermal preparations used for chronic pain).[47]
Buprenorphine has been introduced in most European countries as a transdermal formulation (marketed as Transtec) for the treatment of chronic pain not responding to non-opioids.
Veterinary medicine[edit]
It has veterinary medical use for treatment of pain in dogs and cats.[59][60]
Research[edit]
Depression[edit]
Some evidence supports the use of buprenorphine for depression.[61]Buprenorphine/samidorphan, a combination product of buprenorphine and samidorphan (a preferential μ-opioid receptor antagonist), appears useful for treatment-resistant depression.[62]
Cocaine dependence[edit]
In combination with samidorphan or naltrexone (μ-opioid receptor antagonists), buprenorphine is under investigation for the treatment of cocaine dependence, and recently demonstrated effectiveness for this indication in a large-scale (n = 302) clinical trial (at a high buprenorphine dose of 16 mg but not a low dose of 4 mg).[63][64]
Neonatal abstinence[edit]
Buprenorphine has been used in the treatment of the neonatal abstinence syndrome,[65] a condition in which newborns exposed to opioids during pregnancy demonstrate signs of withdrawal.[66] Use currently is limited to infants enrolled in a clinical trial conducted under an FDA approved investigational new drug (IND) application.[67] An ethanolic formulation used in neonates is stable at room temperature for at least 30 days.[68]
Obsessive–compulsive disorder[edit]
In one study, buprenorphine was found to be effective in a subset of individuals with treatment-refractory obsessive–compulsive disorder.[69]
References[edit]
^ Mendelson J, Upton RA, Everhart ET, Jacob P 3rd, Jones RT (1997). "Bioavailability of sublingual buprenorphine". Journal of Clinical Pharmacology. 37 (1): 31–7. doi:10.1177/009127009703700106. PMID 9048270..mw-parser-output cite.citation{font-style:inherit}.mw-parser-output q{quotes:"""""""'""'"}.mw-parser-output code.cs1-code{color:inherit;background:inherit;border:inherit;padding:inherit}.mw-parser-output .cs1-lock-free a{background:url("//upload.wikimedia.org/wikipedia/commons/thumb/6/65/Lock-green.svg/9px-Lock-green.svg.png")no-repeat;background-position:right .1em center}.mw-parser-output .cs1-lock-limited a,.mw-parser-output .cs1-lock-registration a{background:url("//upload.wikimedia.org/wikipedia/commons/thumb/d/d6/Lock-gray-alt-2.svg/9px-Lock-gray-alt-2.svg.png")no-repeat;background-position:right .1em center}.mw-parser-output .cs1-lock-subscription a{background:url("//upload.wikimedia.org/wikipedia/commons/thumb/a/aa/Lock-red-alt-2.svg/9px-Lock-red-alt-2.svg.png")no-repeat;background-position:right .1em center}.mw-parser-output .cs1-subscription,.mw-parser-output .cs1-registration{color:#555}.mw-parser-output .cs1-subscription span,.mw-parser-output .cs1-registration span{border-bottom:1px dotted;cursor:help}.mw-parser-output .cs1-hidden-error{display:none;font-size:100%}.mw-parser-output .cs1-visible-error{font-size:100%}.mw-parser-output .cs1-subscription,.mw-parser-output .cs1-registration,.mw-parser-output .cs1-format{font-size:95%}.mw-parser-output .cs1-kern-left,.mw-parser-output .cs1-kern-wl-left{padding-left:0.2em}.mw-parser-output .cs1-kern-right,.mw-parser-output .cs1-kern-wl-right{padding-right:0.2em}
^ Eriksen J, Jensen NH, Kamp-Jensen M, Bjarnø H, Friis P, Brewster D (1989). "The systemic availability of buprenorphine administered by nasal spray". J. Pharm. Pharmacol. 41 (11): 803–5. doi:10.1111/j.2042-7158.1989.tb06374.x. PMID 2576057.
^ abcdefghijklmnopqrst "Buprenorphine Hydrochloride". drugs.com. American Society of Health-System Pharmacists. 26 January 2017. Retrieved 17 March 2017.
^ "Press Announcements - FDA approves first buprenorphine implant for treatment of opioid dependence". FDA. Retrieved 12 December 2017.
^ abcd Drug Enforcement Administration (July 2013). "Buprenorphine" (PDF). DEA. Retrieved 3 December 2017.
^ "NADAC as of 2017-11-29". Centers for Medicare and Medicaid Services. Retrieved 3 December 2017.
^ abc "Buprenorphine". www.samhsa.gov. 31 May 2016. Retrieved 3 December 2017.
^ "Press Announcements - FDA approves first once-monthly buprenorphine injection, a medication-assisted treatment option for opioid use disorder". www.fda.gov. Retrieved 5 December 2017.
^ "Indivior drug to fight opioid addiction approved by U.S. FDA". Reuters. 2017. Retrieved 5 December 2017.
^ Gowing, L; Ali, R; White, JM; Mbewe, D (21 February 2017). "Buprenorphine for managing opioid withdrawal". The Cochrane Database of Systematic Reviews. 2: CD002025. doi:10.1002/14651858.CD002025.pub5. PMID 28220474.
^ "Butrans Medication Guide". Butrans Medication Guide. Purdue Pharma L.P. Retrieved 7 July 2014.
^ Nutt, D; King, LA; Saulsbury, W; Blakemore, C (24 March 2007). "Development of a rational scale to assess the harm of drugs of potential misuse". Lancet. 369 (9566): 1047–53. doi:10.1016/S0140-6736(07)60464-4. PMID 17382831.
^ Budd K, Raffa RB. (eds.) Buprenorphine – The unique opioid analgesic. Thieme, 200,
ISBN 3-13-134211-0
^ White et.al. (13 December 2017). "The efficacy and adverse effects of buprenorphine in acute pain management: a systematic review and meta-analysis of randomised controlled trials". British Journal of Anaesthesia. doi:10.1016/j.bja.2017.11.086.
^ Alford, Daniel P.; Compton, Peggy; Samet, Jeffrey H. (17 January 2006). "Acute Pain Management for Patients Receiving Maintenance Methadone or Buprenorphine Therapy". Annals of Internal Medicine. 144 (2): 127–134. doi:10.7326/0003-4819-144-2-200601170-00010. ISSN 0003-4819. PMC 1892816. PMID 16418412.
^ Roth, BL; Driscol, J. "PDSP Ki Database". Psychoactive Drug Screening Program (PDSP). University of North Carolina at Chapel Hill and the United States National Institute of Mental Health. Retrieved 14 August 2017.
^ abc Toll L, Berzetei-Gurske IP, Polgar WE, Brandt SR, Adapa ID, Rodriguez L, Schwartz RW, Haggart D, O'Brien A, White A, Kennedy JM, Craymer K, Farrington L, Auh JS (1998). "Standard binding and functional assays related to medications development division testing for potential cocaine and opiate narcotic treatment medications". NIDA Res. Monogr. 178: 440–66. PMID 9686407.
^ ab Khroyan TV, Polgar WE, Jiang F, Zaveri NT, Toll L (2009). "Nociceptin/orphanin FQ receptor activation attenuates antinociception induced by mixed nociceptin/orphanin FQ/mu-opioid receptor agonists". J. Pharmacol. Exp. Ther. 331 (3): 946–53. doi:10.1124/jpet.109.156711. PMC 2784721. PMID 19713488.
^ abcde Khroyan TV, Wu J, Polgar WE, Cami-Kobeci G, Fotaki N, Husbands SM, Toll L (2015). "BU08073 a buprenorphine analogue with partial agonist activity at μ-receptors in vitro but long-lasting opioid antagonist activity in vivo in mice". Br. J. Pharmacol. 172 (2): 668–80. doi:10.1111/bph.12796. PMC 4292977. PMID 24903063.
^ abc Negus SS, Bidlack JM, Mello NK, Furness MS, Rice KC, Brandt MR (2002). "Delta opioid antagonist effects of buprenorphine in rhesus monkeys". Behav Pharmacol. 13 (7): 557–70. doi:10.1097/00008877-200211000-00005. PMID 12409994.
^ abcd Lutfy K, Cowan A (2004). "Buprenorphine: a unique drug with complex pharmacology". Curr Neuropharmacol. 2 (4): 395–402. doi:10.2174/1570159043359477. PMC 2581407. PMID 18997874.
^ Freye, Enno (1987). "Interaction of Mixed Agonist-Antagonists with Different Receptor Sites Using Nalbuphine as a Model Substance": 67–78. doi:10.1007/978-3-642-71854-0_6.
^ ab Hutchinson MR, Zhang Y, Shridhar M, Evans JH, Buchanan MM, Zhao TX, Slivka PF, Coats BD, Rezvani N, Wieseler J, Hughes TS, Landgraf KE, Chan S, Fong S, Phipps S, Falke JJ, Leinwand LA, Maier SF, Yin H, Rice KC, Watkins LR (2010). "Evidence that opioids may have toll-like receptor 4 and MD-2 effects". Brain Behav. Immun. 24 (1): 83–95. doi:10.1016/j.bbi.2009.08.004. PMC 2788078. PMID 19679181.
^ ab Codd EE, Shank RP, Schupsky JJ, Raffa RB (1995). "Serotonin and norepinephrine uptake inhibiting activity of centrally acting analgesics: structural determinants and role in antinociception". J. Pharmacol. Exp. Ther. 274 (3): 1263–70. PMID 7562497.
^ ab Leffler A, Frank G, Kistner K, Niedermirtl F, Koppert W, Reeh PW, Nau C (2012). "Local anesthetic-like inhibition of voltage-gated Na(+) channels by the partial μ-opioid receptor agonist buprenorphine". Anesthesiology. 116 (6): 1335–46. doi:10.1097/ALN.0b013e3182557917. PMID 22504149.
^ Lutfy, K., & Cowan, A. (2004). Buprenorphine: A Unique Drug with Complex Pharmacology. Current Neuropharmacology, 2(4), 395–402. doi:10.2174/1570159043359477
^ ab Benzon, Honorio; Raja, Srinivasa N.; Fishman, Scott M.; Liu, Spencer S.; Cohen, Steven P. (2017). Essentials of Pain Medicine E-Book. Elsevier Health Sciences. p. 382. ISBN 9780323445412.
^ Jacob JJ, Michaud GM, Tremblay EC (1979). "Mixed agonist-antagonist opiates and physical dependence". Br J Clin Pharmacol. 7 Suppl 3: 291S–296S. doi:10.1111/j.1365-2125.1979.tb04703.x. PMC 1429306. PMID 572694.
^ Kress HG (March 2009). "Clinical update on the pharmacology, efficacy and safety of transdermal buprenorphine". Eur J Pain. 13 (3): 219–30. doi:10.1016/j.ejpain.2008.04.011. PMID 18567516.
^ Robinson SE (2002). "Buprenorphine: an analgesic with an expanding role in the treatment of opioid addiction". CNS Drug Rev. 8 (4): 377–90. PMID 12481193.
^ Pedro Ruiz; Eric C. Strain (2011). Lowinson and Ruiz's Substance Abuse: A Comprehensive Textbook. Lippincott Williams & Wilkins. p. 439. ISBN 978-1-60547-277-5.
^ Bidlack JM (2014). "Mixed kappa/mu partial opioid agonists as potential treatments for cocaine dependence". Adv. Pharmacol. Advances in Pharmacology. 69: 387–418. doi:10.1016/B978-0-12-420118-7.00010-X. ISBN 9780124201187. PMID 24484983.
^ Ehrich, Elliot; Turncliff, Ryan; Du, Yangchun; Leigh-Pemberton, Richard; Fernandez, Emilio; Jones, Reese; Fava, Maurizio (2014). "Evaluation of Opioid Modulation in Major Depressive Disorder". Neuropsychopharmacology. 40 (6): 1448–55. doi:10.1038/npp.2014.330. ISSN 0893-133X. PMC 4397403. PMID 25518754.
^ abc Coller JK, Christrup LL, Somogyi AA (2009). "Role of active metabolites in the use of opioids". Eur. J. Clin. Pharmacol. 65 (2): 121–39. doi:10.1007/s00228-008-0570-y. PMID 18958460.
^ Mizoguchi H, Wu HE, Narita M, et al. (2002). "Antagonistic property of buprenorphine for putative epsilon-opioid receptor-mediated G-protein activation by beta-endorphin in pons/medulla of the mu-opioid receptor knockout mouse". Neuroscience. 115 (3): 715–21. doi:10.1016/s0306-4522(02)00486-4. PMID 12435410.
^ Mizoguchi H, Spaulding A, Leitermann R, Wu HE, Nagase H, Tseng LF (July 2003). "Buprenorphine blocks epsilon- and micro-opioid receptor-mediated antinociception in the mouse". J. Pharmacol. Exp. Ther. 306 (1): 394–400. doi:10.1124/jpet.103.048835. PMID 12721333.
^ Xu J, Xu M, Hurd YL, Pasternak GW, Pan YX (2009). "Isolation and characterization of new exon 11-associated N-terminal splice variants of the human mu opioid receptor gene". J. Neurochem. 108 (4): 962–72. doi:10.1111/j.1471-4159.2008.05833.x. PMC 2727151. PMID 19077058.
^ Grinnell S et al. (2014): Buprenorphine analgesia requires exon 11-associated mu opioid receptor splice variants. The FASEB Journal
^ Harold E. Doweiko (14 March 2014). Concepts of Chemical Dependency. Cengage Learning. pp. 149–. ISBN 978-1-285-45717-8.
^ USP DI. United States Pharmacopeial Convention.
^ Moody DE, Fang WB, Lin SN, Weyant DM, Strom SC, Omiecinski CJ (2009). "Effect of Rifampin and Nelfinavir on the Metabolism of Methadone and Buprenorphine in Primary Cultures of Human Hepatocytes". Drug Metabolism and Disposition. 37 (12): 2323–2329. doi:10.1124/dmd.109.028605. PMC 2784702. PMID 19773542.
^ Yassen A, Kan J, Olofsen E, Suidgeest E, Dahan A, Danhof M (2007). "Pharmacokinetic-pharmacodynamic modeling of the respiratory depressant effect of norbuprenorphine in rats". The Journal of Pharmacology and Experimental Therapeutics. 321 (2): 598–607. doi:10.1124/jpet.106.115972. PMID 17283225.
^ Huang P, Kehner GB, Cowan A, Liu-Chen LY (2001). "Comparison of pharmacological activities of buprenorphine and norbuprenorphine: Norbuprenorphine is a potent opioid agonist". The Journal of Pharmacology and Experimental Therapeutics. 297 (2): 688–695. PMID 11303059.
^ abc Brown SM, Campbell SD, Crafford A, Regina KJ, Holtzman MJ, Kharasch ED (October 2012). "P-glycoprotein is a major determinant of norbuprenorphine brain exposure and antinociception". J. Pharmacol. Exp. Ther. 343 (1): 53–61. doi:10.1124/jpet.112.193433. PMC 3464040. PMID 22739506.
^ Brown SM, Holtzman M, Kim T, Kharasch ED (2011). "Buprenorphine metabolites, buprenorphine-3-glucuronide and norbuprenorphine-3-glucuronide, are biologically active". Anesthesiology. 115 (6): 1251–60. doi:10.1097/ALN.0b013e318238fea0. PMC 3560935. PMID 22037640.
^ Heel RC, Brogden RN, Speight TM, Avery GS (February 1979). "Buprenorphine: a review of its pharmacological properties and therapeutic efficacy". Drugs. 17 (2): 81–110. doi:10.2165/00003495-197917020-00001. PMID 378645.
^ abc "Buprenorphine". Martindale: The Complete Drug Reference. London, UK: Pharmaceutical Press. 14 January 2014. Retrieved 6 April 2014.
^ Baselt, R. (2008). Disposition of Toxic Drugs and Chemicals in Man (8th ed.). Foster City, CA: Biomedical Publications. pp. 190–192. ISBN 0962652377.
^ "IMPORTANT SAFETY INFORMATION".
^ Campbell N. D.; Lovell A. M. (2012). "The history of the development of buprenorphine as an addiction therapeutic". Annals of the New York Academy of Sciences. 1248: 124–139. Bibcode:2012NYASA1248..124C. doi:10.1111/j.1749-6632.2011.06352.x. PMID 22256949.
^ Louis S. Harris, ed. (1998). Problems of Drug Dependence, 1998: Proceedings of the 66th Annual Scientific Meeting, The College on Problems of Drug Dependence, Inc (PDF). NIDA Research Monograph 179.
^ Subutex and Suboxone Approval Letter. U.S. Food and Drug Administration (October 8, 2002). fda.gov.
^ Quotas – Conversion Factors for Controlled Substances. Deadiversion.usdoj.gov. Retrieved on 2016-11-07.
^ "Drug Addiction Treatment Act of 2000" Archived 2013-03-04 at the Wayback Machine.. SAMHSA, U.S. Department of Health & Human Services.
^ The National Alliance of Advocates for Buprenorphine Treatment. naabt.org. Retrieved on 2013-05-19.
^ Obama administration's change on buprenorphine policy. Business Insider (2016-07-06). Retrieved on 2016-11-07.
^ Practically a book review: Dying to be Free. Slate Star Codex. Retrieved June 2015
^ Suboxone EU Approval. Ema.europa.eu. Retrieved on 2016-11-07.
^ Claude, Andrew (June 2015). "Buprenorphine" (PDF). cliniciansbrief.com. Retrieved 25 February 2017.
^ Kukanich, Butch; Papich, Mark G. (May 14, 2013). "Opioid Analgesic Drugs". In Jim E. Riviere, Mark G. Papich. Veterinary Pharmacology and Therapeutics (9 ed.). John Wiley & Sons. pp. 323–325. ISBN 9781118685907.
^ Stanciu, CN; Glass, OM; Penders, TM (April 2017). "Use of Buprenorphine in treatment of refractory depression-A review of current literature". Asian journal of psychiatry. 26: 94–98. doi:10.1016/j.ajp.2017.01.015. PMID 28483102.
^ Ragguett, RM; Rong, C; Rosenblat, JD; Ho, RC; McIntyre, RS (April 2018). "Pharmacodynamic and pharmacokinetic evaluation of buprenorphine + samidorphan for the treatment of major depressive disorder". Expert opinion on drug metabolism & toxicology. 14 (4): 475–482. doi:10.1080/17425255.2018.1459564. PMID 29621905.
^ Ling, Walter; Hillhouse, Maureen P.; Saxon, Andrew J.; Mooney, Larissa J.; Thomas, Christie M.; Ang, Alfonso; Matthews, Abigail G.; Hasson, Albert; Annon, Jeffrey; Sparenborg, Steve; Liu, David S.; McCormack, Jennifer; Church, Sarah; Swafford, William; Drexler, Karen; Schuman, Carolyn; Ross, Stephen; Wiest, Katharina; Korthuis, Philip; Lawson, William; Brigham, Gregory S.; Knox, Patricia C.; Dawes, Michael; Rotrosen, John (2016). "Buprenorphine + Naloxone plus Naltrexone for the Treatment of Cocaine Dependence:The Cocaine Use Reduction with Buprenorphine(CURB)Study". Addiction. 111 (8): 1416–1427. doi:10.1111/add.13375. ISSN 0965-2140. PMC 4940267. PMID 26948856.
^ Reuters (2012). "Alkermes Presents Positive Clinical Data of ALKS 5461 at 52nd Annual New Clinical Drug Evaluation Unit Meeting".
^ Kraft WK, Gibson E, Dysart K, Damle VS, Larusso JL, Greenspan JS, Moody DE, Kaltenbach K, Ehrlich ME (September 2008). "Sublingual buprenorphine for treatment of neonatal abstinence syndrome: a randomized trial". Pediatrics. 122 (3): e601–7. doi:10.1542/peds.2008-0571. PMC 2574639. PMID 18694901.
^ Kraft WK, van den Anker JN (2012). "Pharmacologic Management of the Opioid Neonatal Abstinence Syndrome". Pediatric Clinics of North America. 59 (5): 1147–1165. doi:10.1016/j.pcl.2012.07.006. PMC 4709246. PMID 23036249.
^ Buprenorphine for the Treatment of Neonatal Abstinence Syndrome. Clinicaltrials.gov. NCT00521248. Retrieved on 2013-05-19.
^ Anagnostis EA, Sadaka RE, Sailor LA, Moody DE, Dysart KC, Kraft WK (2011). "Formulation of buprenorphine for sublingual use in neonates". The journal of pediatric pharmacology and therapeutics. 16 (4): 281–284. doi:10.5863/1551-6776-16.4.281. PMC 3385042. PMID 22768012.
^ Liddell, M. B.; Aziz, V.; Briggs, P.; Kanakkehewa, N.; Rawi, O. (2012). "Buprenorphine augmentation in the treatment of refractory obsessive-compulsive disorder". Therapeutic Advances in Psychopharmacology. 3 (1): 15–19. doi:10.1177/2045125312462233. ISSN 2045-1253. PMC 3736962. PMID 23983988.
External links[edit]
- U.S. Federal government buprenorphine program for opioid addiction
- Australian national buprenorphine policy
"The bitter pill": A Wired magazine article on Suboxone
"Subu Must Die – How a nation of junkies went cold turkey": A New Republic article on Subutex abuse in the nation of Georgia]
Categories:
- Alcohols
- Cat medications
- Dog medications
- Delta-opioid antagonists
- Drug rehabilitation
- Ethers
- Euphoriants
- Kappa antagonists
- Morphinans
- Mu-opioid agonists
- Nociceptin receptor agonists
- Nociceptin receptor antagonists
- Oripavines
- Phenols
- Semisynthetic opioids
- Sodium channel blockers
(window.RLQ=window.RLQ||).push(function(){mw.config.set({"wgPageParseReport":{"limitreport":{"cputime":"1.540","walltime":"1.821","ppvisitednodes":{"value":11687,"limit":1000000},"ppgeneratednodes":{"value":0,"limit":1500000},"postexpandincludesize":{"value":532965,"limit":2097152},"templateargumentsize":{"value":14144,"limit":2097152},"expansiondepth":{"value":16,"limit":40},"expensivefunctioncount":{"value":7,"limit":500},"unstrip-depth":{"value":1,"limit":20},"unstrip-size":{"value":192732,"limit":5000000},"entityaccesscount":{"value":5,"limit":400},"timingprofile":["100.00% 1334.571 1 -total"," 45.45% 606.542 1 Template:Reflist"," 32.87% 438.630 1 Template:Drugbox"," 28.90% 385.708 38 Template:Cite_journal"," 25.00% 333.661 1 Template:Infobox"," 14.53% 193.875 26 Template:Navbox"," 8.19% 109.323 16 Template:Unbulleted_list"," 6.29% 83.914 72 Template:Abbr"," 4.50% 60.010 1 Template:Ion_channel_modulators"," 4.08% 54.502 44 Template:Abbrlink"]},"scribunto":{"limitreport-timeusage":{"value":"0.658","limit":"10.000"},"limitreport-memusage":{"value":7500993,"limit":52428800}},"cachereport":{"origin":"mw1263","timestamp":"20181105225347","ttl":1900800,"transientcontent":false}}});mw.config.set({"wgBackendResponseTime":113,"wgHostname":"mw1254"});});

 Clash Royale CLAN TAG
Clash Royale CLAN TAG