Zolmitriptan
Zolmitriptan
Jump to navigation
Jump to search
This article needs additional citations for verification. (August 2009) (Learn how and when to remove this template message) |
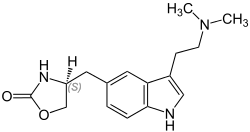 | |
| Clinical data | |
|---|---|
| Trade names | Zomig |
AHFS/Drugs.com | Monograph |
| Pregnancy category |
|
| Routes of administration | Oral, nasal spray |
| ATC code |
|
| Legal status | |
| Legal status |
|
Pharmacokinetic data | |
| Bioavailability | 40% (oral) |
| Protein binding | 25% |
| Metabolism | Hepatic (CYP1A2-mediated, to active metabolite) |
| Elimination half-life | 3 hours |
| Excretion | Renal (65%) and fecal (35%) |
| Identifiers | |
IUPAC name
| |
| CAS Number |
|
PubChem CID |
|
| IUPHAR/BPS |
|
| DrugBank |
|
| ChemSpider |
|
| UNII |
|
| KEGG |
|
| ChEBI |
|
| ChEMBL |
|
| ECHA InfoCard | 100.158.186 |
| Chemical and physical data | |
| Formula | C16H21N3O2 |
| Molar mass | 287.357 g/mol |
| 3D model (JSmol) |
|
SMILES
| |
InChI
| |
.mw-parser-output .nobold{font-weight:normal} (verify) | |
Zolmitriptan is a selective serotonin receptor agonist of the 1B and 1D subtypes. It is a triptan, used in the acute treatment of migraine attacks with or without aura and cluster headaches.
Zolmitriptan is marketed by AstraZeneca with the brand names Zomig, Zomigon (Argentina, Canada & Greece), AscoTop (Germany) and Zomigoro (France). In 2008, Zomig generated nearly $154 million in sales.[1]
AstraZeneca's U.S. patent on Zomig tablets expired on November 14, 2012, and its pediatric exclusivity extension expired on May 14, 2013.[2] The patent in certain European countries has already expired too, and generic drug maker Actavis released a generic version in those countries, starting in March 2012.[3]
Contents
1 Description
2 Indications
3 Contraindications and precautions
4 Adverse reactions
5 References
6 External links
Description[edit]
Zolmitriptan is a synthetic tryptamine derivative and appears as a white powder that is partially soluble in water.
Indications[edit]
Zolmitriptan is used for the acute treatment of migraines with or without aura in adults. Zolmitriptan is not intended for the prophylactic therapy of migraine or for use in the management of hemiplegic or basilar migraine.
Zolmitriptan is available as a swallowable tablet, an oral disintegrating tablet, and a nasal spray, in doses of 2.5 and 5 mg. People who get migraines from aspartame should not use the disintegrating tablet (Zomig ZMT), which contains aspartame.[4]
According to a study of healthy volunteers, food intake seems to have no significant effect on the effectiveness of Zolmitriptan in both men and women.[5]
Contraindications and precautions[edit]
Zolmitriptan should not be given to patients with ischemic heart disease (angina pectoris, history of myocardial infarction, or documented silent ischemia) or to patients who have symptoms or findings consistent with ischemic heart disease, coronary artery vasospasm, including Prinzmetal's angina, or other significant underlying cardiovascular disease.
Zolmitriptan may increase blood pressure, it should not be given to patients with uncontrolled hypertension, should not be used within 24 hours of treatment with another 5-HT1 agonist, or an ergotamine-containing or ergot-type medication like dihydroergotamine or methysergide, and should not be administered to patients with hemiplegic or basilar migraine.
Concurrent administration of MAOI or use of zolmitriptan within 2 weeks of discontinuation of MAO-A inhibitor therapy is contraindicated.
Adverse reactions[edit]
The Zomig ZMT dissolvable pill contains aspartame, and should be avoided by anyone sensitive to that ingredient and by those suffering from phenylketonuria.
Rarely, serious cardiac events, including myocardial infarction (heart attack), have been associated with zolmitriptan.
Reported minor adverse reactions include: hypesthesia, paresthesia (all types), warm and cold sensations, chest pain, throat and jaw tightness, dry mouth, dyspepsia, dysphagia, nausea, somnolence, vertigo, asthenia, myalgia, myasthenia and sweating.
Drug Interactions: see Zomig package insert for complete list: [1]
Following administration of cimetidine, the half-life and AUC of zolmitriptan and its active metabolites were approximately doubled (see CLINICAL PHARMACOLOGY in product pamphlet). Cimetidine (INN) (/sɪˈmɛtɪdiːn/ or /saɪˈmɛtɪdiːn/) is a histamine H2-receptor antagonist that inhibits the production of acid in the stomach.
References[edit]
^ ""2008 Top 200 generic drugs by retail dollars"" (PDF). Archived from the original (PDF) on 2009-05-21..mw-parser-output cite.citation{font-style:inherit}.mw-parser-output q{quotes:"""""""'""'"}.mw-parser-output code.cs1-code{color:inherit;background:inherit;border:inherit;padding:inherit}.mw-parser-output .cs1-lock-free a{background:url("//upload.wikimedia.org/wikipedia/commons/thumb/6/65/Lock-green.svg/9px-Lock-green.svg.png")no-repeat;background-position:right .1em center}.mw-parser-output .cs1-lock-limited a,.mw-parser-output .cs1-lock-registration a{background:url("//upload.wikimedia.org/wikipedia/commons/thumb/d/d6/Lock-gray-alt-2.svg/9px-Lock-gray-alt-2.svg.png")no-repeat;background-position:right .1em center}.mw-parser-output .cs1-lock-subscription a{background:url("//upload.wikimedia.org/wikipedia/commons/thumb/a/aa/Lock-red-alt-2.svg/9px-Lock-red-alt-2.svg.png")no-repeat;background-position:right .1em center}.mw-parser-output .cs1-subscription,.mw-parser-output .cs1-registration{color:#555}.mw-parser-output .cs1-subscription span,.mw-parser-output .cs1-registration span{border-bottom:1px dotted;cursor:help}.mw-parser-output .cs1-hidden-error{display:none;font-size:100%}.mw-parser-output .cs1-visible-error{font-size:100%}.mw-parser-output .cs1-subscription,.mw-parser-output .cs1-registration,.mw-parser-output .cs1-format{font-size:95%}.mw-parser-output .cs1-kern-left,.mw-parser-output .cs1-kern-wl-left{padding-left:0.2em}.mw-parser-output .cs1-kern-right,.mw-parser-output .cs1-kern-wl-right{padding-right:0.2em} (332 KB). Drug Topics (May 26, 2009). Retrieved on August 25, 2009.
^ https://www.drugs.com/availability/generic-zomig-zmt.html
^ http://www.actavis.com/en/media+center/newsroom/articles/migraine_zolmitriptan_eu.htm
^ Newman LC, Lipton RB (2001). "Migraine MLT-Down: An Unusual Presentation of Migraine in Patients With Aspartame-Triggered Headaches". Headache: The Journal of Head and Face Pain (abstract). 41 (9): 899–901. doi:10.1046/j.1526-4610.2001.01164.x.
^ Emma J. Seaber; Richard W. Peck; Deborah A. Smith; John Allanson; Nanco R. Hefting; Jan J. van Lier; Frans A.E. Sollie; Johan Wemer; Jan H.G. Jonkman (1998). "The absolute bioavailability and effect of food on the pharmacokinetics of zolmitriptan in healthy volunteers". British Journal of Clinical Pharmacology (abstract). 46 (5): 433–439. doi:10.1046/j.1365-2125.1998.00809.x. PMC 1873688.
MacGregor, E.A. (1998). "Zolmitriptan clinical studies". Drugs Today. 34 (12): 1027–1033. doi:10.1358/dot.1998.34.12.487488. PMID 14743270.
External links[edit]
- Zomig Information Site (USA)
Categories:
- Triptans
- Oxazolidinones
- AstraZeneca
- 5-HT1D agonists
(window.RLQ=window.RLQ||).push(function(){mw.config.set({"wgPageParseReport":{"limitreport":{"cputime":"0.820","walltime":"1.051","ppvisitednodes":{"value":5354,"limit":1000000},"ppgeneratednodes":{"value":0,"limit":1500000},"postexpandincludesize":{"value":341259,"limit":2097152},"templateargumentsize":{"value":5156,"limit":2097152},"expansiondepth":{"value":14,"limit":40},"expensivefunctioncount":{"value":4,"limit":500},"unstrip-depth":{"value":1,"limit":20},"unstrip-size":{"value":11907,"limit":5000000},"entityaccesscount":{"value":3,"limit":400},"timingprofile":["100.00% 724.687 1 -total"," 58.70% 425.390 1 Template:Drugbox"," 43.95% 318.520 1 Template:Infobox"," 22.49% 162.996 22 Template:Navbox"," 14.01% 101.562 16 Template:Unbulleted_list"," 9.06% 65.634 1 Template:Cite_web"," 8.74% 63.308 1 Template:More_citations_needed"," 7.99% 57.932 1 Template:Serotonergics"," 6.36% 46.059 1 Template:Ambox"," 5.17% 37.480 3 Template:Cite_journal"]},"scribunto":{"limitreport-timeusage":{"value":"0.267","limit":"10.000"},"limitreport-memusage":{"value":5809984,"limit":52428800}},"cachereport":{"origin":"mw1254","timestamp":"20181222041602","ttl":1900800,"transientcontent":false}}});});{"@context":"https://schema.org","@type":"Article","name":"Zolmitriptan","url":"https://en.wikipedia.org/wiki/Zolmitriptan","sameAs":"http://www.wikidata.org/entity/Q218820","mainEntity":"http://www.wikidata.org/entity/Q218820","author":{"@type":"Organization","name":"Contributors to Wikimedia projects"},"publisher":{"@type":"Organization","name":"Wikimedia Foundation, Inc.","logo":{"@type":"ImageObject","url":"https://www.wikimedia.org/static/images/wmf-hor-googpub.png"}},"datePublished":"2004-11-07T18:30:14Z","dateModified":"2018-11-16T02:50:12Z","image":"https://upload.wikimedia.org/wikipedia/commons/7/71/Zolmitriptan_Structure_V.1.svg","headline":"chemical compound"}(window.RLQ=window.RLQ||).push(function(){mw.config.set({"wgBackendResponseTime":110,"wgHostname":"mw1257"});});

